Targeting the retinoid signaling pathway with YCT-529 for effective and reversible oral contraception in mice and primates

Introduction
Since nearly half of all pregnancies in the US and worldwide are unintended, a critical need exists for additional contraceptive options applicable throughout the reproductive lifespans of both women and men1,2,3. Women have multiple contraceptive options, but men are limited to condoms and vasectomy4. Testosterone and testosterone derivatives have been clinically evaluated for male contraception; however, none have been marketed5. The importance of dietary vitamin A and retinoid signaling for male germ cell development and differentiation has been recognized for many years6. All trans-retinoic acid (Fig. 1a) is an active metabolite of vitamin A that exerts its function, at least partly, by binding to retinoic acid receptors (RARs). The RARs α, β, and γ, are encoded by the Rara, Rarb, and Rarg genes in mice, and Rarα and Rarγ have been validated as contraceptive targets by genetic knockouts resulting in male sterility7,8. Notably, the effects on spermatogenesis in the absence of RARα most resemble the loss of RAR signaling in vitamin A deficiency, and the mice are otherwise normal7,8. Further, the effects on spermatogenesis in animals treated orally with the dual RARα/RARγ antagonist BMS-189453 (Fig. 1a, b) closely phenocopied the absence of RARα function. Importantly, the resulting male sterility is reversible9,10,11. We, therefore, wished to identify RARα−selective inhibitors for potential male non-hormonal contraception. Our study describes the development of YCT-529, a highly selective RARα antagonist that reduces sperm counts in mice and non-human primates. Mating studies with male mice treated with 10 mg/kg/day for 4 weeks show that YCT-529 is 99% effective in preventing pregnancies and that the mice fully regain fertility after drug cessation.
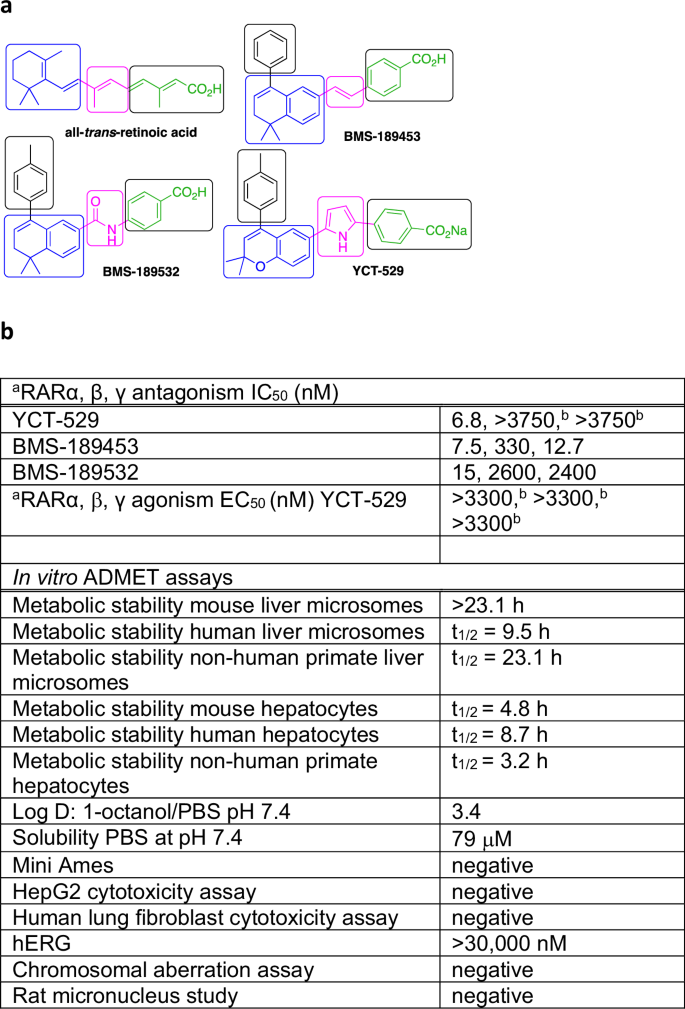
a Structures of agonist all-trans-retinoic acid, antagonists BMS-189453, BMS-189532, and YCT-529. The general ligand scaffold consists of a hydrophobic ring system (blue) and a conjugated acid or a benzoic acid (green) that are connected by a linker (pink). The linker group determines receptor subtype specificity. A large substituent on the hydrophobic ring (black) is required for antagonist activity. b IC50 data for YCT-529, BMS-189453, and BMS-189532, and EC50 data for YCT-529 against RARα, RARβ, and RARγ using transactivation assays. aTransactivation assays. bHighest concentration tested. a,bAverage of at least three independent experiments. The in vitro ADMET assays were performed by Pharmaron, except for the chromosomal aberration assay and the rat micronucleus study, which were conducted by WuXi AppTec.
Methods
Three independent measurements were performed for the transactivation assays. All other measurements were taken from distinct samples, and no sample was measured repeatedly.
Transactivation assay
The Human Retinoic Acid Receptor Reporter Assay System kits (Indigo Biosciences, State College, PA) were used to quantify the potency of test compounds as antagonists of the α, β, and γ RAR subtypes. These proprietary mammalian reporter cells express a recombinant human RAR subtype and a beetle luciferase reporter gene functionally linked to a RAR response element. A dose-response of agonist, 9-cis-retinoic acid (9-cis-RA) for RARα or ATRA for RARβ and RARγ was included on every 384-well plate. Reference compounds BMS-189532 and BMS-189453 were used for RARα, RARβ, or RARγ, respectively, and were also included on every plate. Control wells containing cells without added agonist defined the background signal values. An EC80 concentration of agonist in DMSO (180 nM 9-cis-RA for RARα, 8 nM ATRA for RARβ and RARγ) was added to control and compound wells using the Echo acoustic nanoliter dispenser (Labcyte, San Jose, CA). Test and reference compounds in DMSO (final 0.4%) were added to plates in an 8-point dose response in triplicate. A cell suspension (30 μL, 10000-20000 cells per well) was added to each well, and the assay plate was incubated overnight in a 5% CO2 incubator at 37 °C. Luciferase detection reagent (15 μL) was added, and the plate was incubated at ambient temperature for 30 min. Luminescence was quantified using an EnSpire plate reader (PerkinElmer, Waltham, MA). IC50 and EC50 values were determined by fitting dose-response data using the four-parameter logistic equation in GraphPad Prism 7.0. Mean ± SEM values were calculated from the geometric mean of the log IC50 and EC50 values.
Mouse pharmacokinetic (PK) study
Animals
Male CD-1 mice (6-8 weeks, 30-35 g) were obtained from Envigo (Indianapolis, IN). The University of Minnesota’s Institutional Animal Care and Use Committee (IACUC) approved the study protocol. Mice were acclimated to their home cages for 72 h and received standard food and water ad libitum in a temperature-controlled room (25 °C) with a 12 h on/off light cycle before the start of the study.
Dosing and sample collection
Thirty (30) CD-1 male mice were dosed orally by gavage with 10 mg/kg of YCT-529 in saline. At specified time points (5, 15, 30 min and 1, 2 4, 8, 16, 24, 48 h post dose), sets of 3 animals were bled and euthanized to collect plasma, brain, and testes. Plasma and tissue samples were frozen at <− 20 °C until LC/MS/MS analysis. Processing of brain and testes consisted of weighing samples and adding 2x volume of cold H2O. The tissue mixtures were then homogenized using a Polytron PT 2500E (Kinematica AG, Luzern, Switzerland) with a 5 mm probe (PT-DA 05/2 EC-E85, Kinematica AG). Homogenates were stored at ≤−20 °C until LC/MS/MS analysis.
LC/MS/MS analysis
The analyte (YCT-529) has a free acid MW = 457.52; the internal standard (SM-1-194) has a free acid MW = 449.55. SM-1-194 differs from YCT-529 by the presence of an N-methyl group on the indole moiety. LC/MS/MS analysis was performed using a Quattro Ultima triple quadrupole mass spectrometer (Waters, Milford, MA) coupled with a Waters Acquity Ultra Performance Liquid Chromatography (UPLC) system. LC was performed using a Synergi Polar-RP column (75 × 2 mm, 4 μm; Phenomenex, Torrance, CA). Elution was isocratic using 25% H20/0.1% formic acid and 75% acetonitrile/0.1% formic acid flowing at 0.35 mL/min. The total run time was 4.0 min. YCT-529 elutes between 1.7–1.9 min, whereas SM-1-194 elutes between 1.0–2.2 min. MS/MS was performed with the instrument operating in electrospray positive ion mode (ES + ). Parent/daughter mass to charge ratio (m/z) transitions were 438.15 → 141.97 and 450.2 → 141.97 for YCT-529 and SM-1-194, respectively.
PK analysis
Pharmacokinetic analyses of plasma YCT-529 concentration–time data were conducted using non-compartmental Model 200 of WinNonlin®, version 5.2 (Pharsight Corporation, St Louis, MO). No data point was excluded from the analysis.
Mouse efficacy study
Animals and dosing
The use of animals was approved by Columbia University Irving Medical Center’s Animal Care and Use Committee. CD1 mice (8 weeks, 30 g body weight) were obtained from Charles River Laboratories (Wilmington, MA). Oral delivery of the drug followed our previously described protocols1,2. Briefly, YCT-529 was suspended in 0.85% saline to obtain the desired concentrations and was administered to CD1 males (n = 10 per time-point; a sample size of 10 males was shown in our previous studies to yield statistically significant and reproducible assessments via oral gavage at a dose of 10 mg/kg/day or vehicle only (n = 5) for 14 days1,3. In subsequent experiments, YCT-529 was suspended in 0.85% saline to obtain the desired concentrations and was administered to CD1 males (n = 20 each group) via oral gavage with a standard daily dose of 10 mg/kg/day or 20 mg/kg/day, which was adjusted weekly for increased weight of the mice for 4 and 2 weeks, respectively, followed our previously described protocols1,3. For both regimens, the mice were observed daily for overall health and behavior changes. Body weights were recorded, and physical examinations were performed weekly.
Gross and histological assessment of testes
The treated males were euthanized at 2 time points (one day and 4 weeks) post-CDT to assess the effect of the compound on spermatogenesis. At the specified time points, testes were dissected from anesthetized mice (which were then euthanized) and weighed. Testicular weight within the same treatment group was assessed by statistical analysis using Student’s paired t-test in GraphPad Analysis Software. One testis was fixed with 4% paraformaldehyde in 1x Phosphate Buffered Saline (PBS) buffer, and the second with Bouin’s fixative overnight at 4 °C. Fixed tissues were embedded in paraffin, sectioned, and mounted as previously described1,3. For staging of testicular tubules4, sections were stained for the Periodic acid-Schiff reaction before hematoxylin counterstaining as previously reported5,6 and examined by bright-field microscopy.
Sperm counts
Sperm were collected into modified Whitten’s medium at 37 °C from both cauda epididymides of the euthanized animals as described previously1,7. Briefly, caudae were minced with small scissors to release the sperm, which were further extruded from the pieces by round forceps. Large cells, tissue fragments, and debris were filtered out via a 100 µm filter. A 1:10 dilution was made, and sperm were counted manually using a hemocytometer.
Assessment of fertility
Fertility was assessed using our well-established procedures3. Briefly, drug-treated males were placed in individual cages with two untreated virgin females of the same strain for 14 days (three estrus cycles elapsed). Females were checked daily for postcoital vaginal plugs. At 14 days, they were euthanized, and the number of pregnant females, implantation sites, viable fetuses, resorptions, and females with resorptions were recorded. Another 2 females were replaced continuously until either fertility was restored (a minimum of 3 consecutive pregnancies were assessed) or the mice reached at least 6 months after CDT. The mice were then euthanized, and the testes were dissected, weighed, and prepared for histological examination as above.
Non-human primate PK and efficacy studies
Animals and husbandry
Six (6) sexually mature, non-naïve male Cynomolgus macaques (8–15 years, 6–15 kg) were used. The animals were individually housed in a humidity- and temperature-controlled facility under a 12 h light/12 h dark diurnal cycle with two daily meals and water ad libitum. The animals were provided with cage toys and daily fresh fruits for environmental enrichment. The animals were acclimated for 2 weeks and observed twice daily to assess overall health and behavior. The animal use protocol was approved by WuXi App Tec’s IACUC per the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guideline. The conduct of the study followed the Guidance for the Care and Use of Laboratory Animals (NRC 2011, 8th Edition), with a USDA Category C for the pain and distress level. The humane endpoints included: significant body weight drop of 20% within 7 days, severe adverse effort associated with the administration of the test article, pain and distress caused by any of the in-life procedures, and injury sustained during any time of the experiment that could impact the well-being of the animal (all determined by attending veterinarian). All animals were non-naïve and only animals that had normal hematology and serum chemistry parameters, and normal baseline sperm counts were included in the studies. After the end of the study, all animals were returned to the colony. Confounders were not controlled.
Dosing regimens
YCT-529 was suspended in 0.9% saline to prepare working solutions of desired concentrations, which were administered to the animals via oral gavage at a dosing volume of 5 mL/kg. The formulation (YCT-529 in saline) was prepared freshly each day of dosing. Single dose administration: Three (3) animals received a single dose of 1, 5, and 10 mg/kg of YCT-529 with a 7-day washout period between doses. Repeat dose administration: Three (3) Cohort 1 animals were dosed at 0.5 mg/kg/day (Day 1-46) followed by 1.5 mg/kg/day (Day 47–51), 2.5 mg/kg/day (Day 52–101) and 5 mg/kg/day (Day 102-108). Three (3) Cohort 2 animals were dosed at 5 mg/kg/day (Day 1–30) followed by 7.5 mg/kg/day (Day 31–37). No control animals were used in the single and repeat dose studies. Since bioavailability was assessed at time point zero in the PK study and sperm parameters were assessed at pre-dose in the efficacy studies, each animal served as its own control. Animals were not randomized as all animals per group received the same dose levels of YCT-529. No formal sample size calculations were performed as 3 animals per group were considered a sufficient minimum number of animals for this exploratory pilot study to assess bioavailability and efficacy.
PK assessments
In the single dose study and on Days 1 and 28 of the repeat dose studies, blood samples were drawn from all 3 animals at t = 0 (pre-dose) and 0.5, 1, 2, 4, 8, 24, 48, 72, 96, 120, 144 and 168 h post-dose to assess plasma concentrations of YCT-529 over time. Blood samples were collected at single time points on the last day of dosing and at the end of the recovery period in the repeat dose studies. At each time point, approximately 1 mL of whole blood was collected into an EDTA-K2 anticoagulant tube from a vein of the left forelimb for PK analysis. Plasma was separated by centrifugation at 3500 x g for 10 min at 4 °C and stored frozen at –80 °C until LC/MS/MS analysis. No data point was excluded from the analysis. Pharmacokinetic analyses of plasma YCT-529 concentration–time data were conducted using non-compartmental Model 200 of WinNonlin®, version 5.2 (Pharsight Corporation, St Louis, MO).
LC/MS/MS analysis
LC/MS/MS analysis was performed using a LCMS-8060 triple quadrupole mass spectrometer (Shimadzu, Kyoto, Japan) coupled with a Triple Quad 5500+ mass spectrometer (Sciex, Framingham, MA). LC was performed using an InertSustain C18 HP column (50 × 2.1 mm, 3 μm; GL Sciences Inc., Tokyo, Japan). Elution was isocratic using H20/0.1% formic acid and 100% acetonitrile/0.1% formic acid flowing at 0.4 mL/min. The total run time was 4.0 min. YCT-529 eluted at 1.97 min, and glibenclamide (internal standard) at 1.35 min. MS/MS was performed with the instrument operating in electrospray positive ion mode (ES + ).
Semen collection and sperm count/concentration assessment
Semen samples were obtained once per week or once every other week via electroejaculation during the entire repeat dose study periods from all 3 animals per Cohort to assess sperm concentration and count. Samples were left at room temperature for 30 min to allow for liquefication. Liquefied semen was diluted with 3% NaCl solution for manual sperm count assessment using a hemocytometer. All samples were analyzed in triplicates. Sperm concentration per ejaculate was calculated with the following formula: Sperm concentration = Sperm count x 104 x Dilution factor. No data point was excluded from the analysis.
Testicular biopsy collection and histology
Testicular biopsies (2 per animal; one from the right and one from the left testicle) were taken 24 h after administering the last dose and on the last day of the recovery period from all 3 animals per Cohort to assess morphological changes. Preoperative care of animals consisted of food restriction and general examination. All surgical procedures were conducted using aseptic techniques and comprehensive physiologic monitoring. The animals were anesthetized with Zoletil 50 (5 mg/kg) by intramuscular (i.m.) injection. The scrotum was disinfected with iodine and 75% alcohol, and lidocaine (1%) was administered intradermally at the biopsy needle insertion site. Core biopsies (one per testis) were collected with a 16 G TSK starcut biopsy needle. Immediately after biopsy collection, animals received Meloxicam (0.2 mg/kg) and Ketorolac (10 mg) for pain relief, and Ceftriaxone sodium (0.5 g) as an anti-inflammatory (all via i.m. injection). Meloxicam was given once daily for 3 consecutive days, Ketorolac twice daily for 2 days, and Ceftriaxone sodium once daily for 4 days post-collection. Testicular tissue was transferred to Neutral Buffered Formalin (NBF) for subsequent histological preparation. Fixed tissue was embedded in paraffin, and morphological examination was performed with H&E-stained slides.
Clinical pathology and hormone levels
Blood samples were collected at pre-dose, on Day 29, 24 h after administering the last dose, and on the last day of the recovery period from all 3 animals per Cohort to assess hematology and serum chemistry parameters, and hormone levels. Whole blood was collected into EDTA-K2 anticoagulant tubes for complete blood count (CBC) analysis. Whole blood was collected into serum separator tubes for a comprehensive metabolic panel, lipid panel, testosterone, FSH, and inhibin B levels. The serum was separated by centrifugation at 3500 x g for 10 min at 4 °C and stored frozen at –80 °C until analysis. Testosterone, FSH, and inhibin-B levels in serum were analyzed with ELISA. Each sample per animal and time point was measured in duplicates, and the average was calculated. No data point was excluded from the analysis.
Statistics and reproducibility
Three independent measurements were performed for the transactivation assays. All other measurements were taken from distinct samples, and no sample was measured repeatedly.
Mouse experiments: One-way ANOVA statistical analyses were performed for testicular weights to assess changes of drug-treated versus control with Origin 2024 version 10.2.3.403. Per time point, samples from 5 animals, 10 testes were analyzed. NHP experiments: One-way ANOVA statistical analyses were performed for hematology parameters, serum chemistry parameters, and hormone levels to assess changes from baseline with Origin 2022 version 9.9.0.225. Per time point, samples from 3 animals were analyzed.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Development and characterization of YCT-529
Retinoic acid receptor ligands can be agonists, antagonists, or inverse agonists12,13,14. They have been investigated for potential therapeutic applications such as skin disorders, cancer, metabolic diseases, and male contraception13,14,15,16. Typically, a ligand scaffold consists of a hydrophobic ring system and a conjugated acid or a benzoic acid connected by a linker (Fig. 1 a). A large substituent on the hydrophobic ring is required for antagonist activity. The linker group determines receptor subtype specificity. BMS-189453, featuring a double bond linker, is a RARα,γ selective antagonist and a RARβ partial agonist/antagonist10,17. A pyrrole linker can impact RARα selectivity18. Amides, such as BMS-189532 (Fig. 1a, b), are selective for RARα inhibition12. We combined a chromene with a pyrrole linker and benzoic acid18,19, which yielded YCT-529, a potent RARα−selective antagonist (IC50 = 6.8 nM) without agonism at all three receptors (Fig. 1a, b). In vitro ADMET testing (Fig. 1b) demonstrated drug-like properties such as excellent metabolic stability in mouse and human liver microsomes and hepatocytes, a Log D = 3.4, and good solubility. YCT-529 did not inhibit the cardiac hERG ion channel, was not toxic for HepG2 and lung fibroblast cells, and showed neither mutagenic (Ames assay) nor genotoxic (chromosomal aberration and rat micronucleus studies) potential.
Given the favorable characteristics of YCT-529, we determined its in vivo oral bioavailability and pharmacokinetic (PK) properties in plasma, brain and testes of male CD-1 mice (6–8 weeks, 30–35 g). The University of Minnesota’s Institutional Animal Care and Use Committee (IACUC) approved the PK study protocol. After single dose administration of YCT-529 (10 mg/kg in saline), YCT-529 rapidly accumulated in the systemic plasma with peak levels (Cmax) of 907 ng/ml or 2 µM that occurred within 30 min and were 299-fold the IC50 (6.7 nM) for RARα (Fig. 1b). Plasma levels were still above the IC50 for 48 h post-dose (12.9 nM), which led to a calculated systemic half-life (T½) = 6.1 h. YCT-529 accumulated rapidly in the testes and brain. Peak concentrations in the testes (900 ng/g or 2 μM) occurred at 4 h post-dosing compared to 0.5 h in plasma. LC/MS/MS bioanalyses of YCT-529 levels in tissue samples are summarized in Supplementary Fig. 1a, and PK parameters in Supplementary Table 1.
In vivo assessment of the RARα-selective antagonist YCT-529 for inhibiting spermatogenesis
As a first step in establishing the efficacy of this RARα-selective antagonist in inducing infertility, we undertook a ‘proof-of-principle’ study in which YCT-529 was administered orally to CD1 male mice at 10 mg/kg/day for 2 weeks (n = 10). The animal use protocol was approved by Columbia University Irving Medical Center’s Animal Care and Use Committee. CD1 mice (8 weeks, 30 g body weight) were obtained from Charles River Laboratories (Wilmington, MA). Testes were weighed and fixed for histological examination, and caudal epididymal sperm counts were assessed on day one and 4 weeks after cessation of drug treatment (CDT).
One day post-CDT, testicular weight was not affected, but the sperm counts were lower (Supplementary Fig. 2a, b). Reduced numbers of sperm and germ cell sloughing were observed in the epididymis, along with a failure of spermatid translocation and sperm release in 27.5% of stage VIII*-IX* tubules (n = 8 of 10) (Fig. 2a–f) and two males exhibited even more severely disrupted spermatogenesis (Supplementary Fig. 3a–d). Four weeks post-CDT, testicular weights were normal, but sperm counts were still reduced in half of the males (Supplementary Fig. 2a, b). Most testes (n = 8 of 10) exhibited proper spermatid translocation but a failure of sperm release (Supplementary Fig. 3e–g), albeit in fewer stage IX* tubules (8.2%), compared to one day post-CDT.
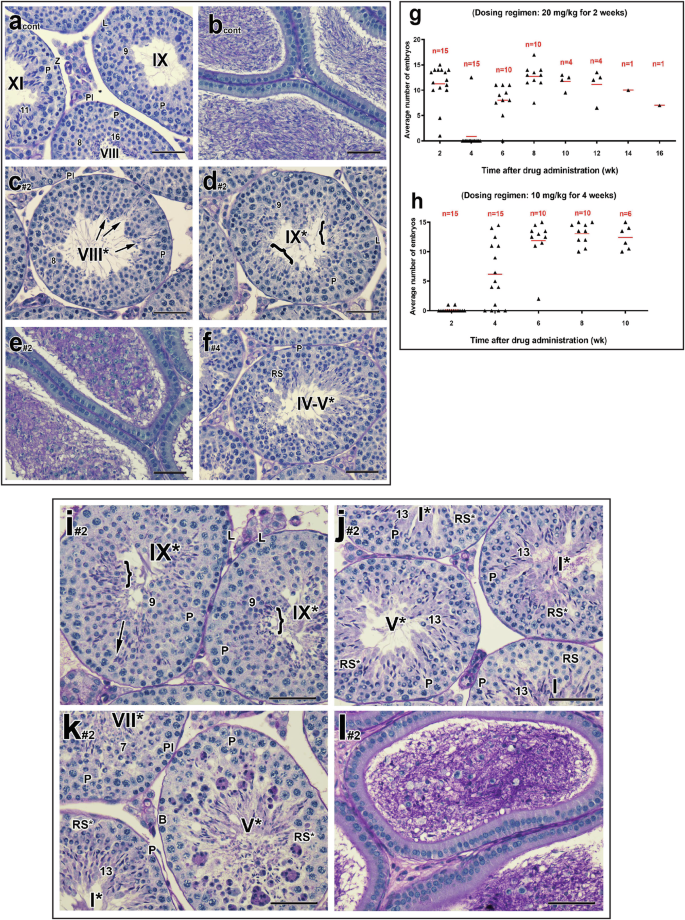
a–f Representative testicular and epididymal histological sections of male mice (n = 8 out of 10) treated with 10 mg/kg/day of YCT-529 for 2 weeks and terminated one day post-CDT. Cont, control sample; Pl, preleptotene spermatocytes; L, leptotene spermatocytes; P, pachytene spermatocytes, RS, round spermatids; Z, zygotene spermatocytes. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the tubules. Although abnormal cell associations complicate staging, an attempt was made to stage the drug-treated tubules using the acrosomal system. Drug-treated tubules are, therefore, labeled with a Roman numeral followed by an asterisk (e.g., stage IX*). Failure of spermatid translocation and sperm release in the tubules. c, d Bracket in stage IX* tubules indicates retained spermatids. Reduced numbers of sperm and germ cell sloughing in the epididymis (e). Scale bar in a-f, 50 µm. Infertility was induced more quickly in male mice treated with 10 mg/kg/day for a longer dosing regimen of YCT-529 of 4 weeks (h) with a full recovery of spermatogenesis, as compared to a higher dose of 20 mg/kg/day for 2 weeks (g). Males used for the mating studies were euthanized at 8–12 weeks post-CDT. They exhibited normal testicular weight and cauda epididymal sperm counts (53.90 × 106 for the mating± 9.34 versus control 55.5 × 106 ± 8.18, n = 10). i–l Representative testicular and epididymal histological sections of male mice treated with 20 mg/kg/day of YCT-529 for 2 weeks and terminated one day post-CDT. Morphological abnormalities included reduced numbers of epididymal sperm and germ cell sloughing, a failure of spermatid translocation (i, #1, 29.3%; #2, 7.5%), abnormal round spermatids (j, #1, 37%; #2, 60.4%), and multinucleated giant cells (k, #1, 1.4%; #2, 12.7%). Many of those abnormal round spermatids showed crescent-like chromatin condensation with juxtanuclear clear spaces (n = 2). Pl, preleptotene spermatocytes; L, leptotene spermatocytes; P, pachytene spermatocytes; B, type B spermatogonia; RS, round spermatids. Brackets in stage IX* tubules indicate retained spermatids. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the tubules. Drug-treated tubules are labeled with a Roman numeral followed by an asterisk (e.g., stage IX*). Scale bar in i-l, 50 µm.
A limited mating study (n = 20 females; 10 males) revealed that three males failed to yield pregnancies after mating for 2 weeks (3-4 weeks post-CDT), indicating YCT-529 could affect fertility. Two of these infertile males exhibited reduced testicular weight and sperm counts (Supplementary Fig. 2), reduced numbers of sperm and germ cell sloughing in the epididymides, and more severely disrupted testicular morphology, with some tubules missing entire layers of specific types of and severe sloughing of spermatogenic cells (Supplementary Fig. 3h–j).
We then undertook a full-scale experiment, including a vehicle control (0.85% saline), and evaluated the above parameters in detail. As expected, oral administration of the saline vehicle alone did not affect spermatogenesis (data not shown). The patterns of changes in testis weight and sperm counts and the morphological abnormalities observed in the pilot study were consistently and reproducibly observed in males one day and 4 weeks post-CDT. Half of the males n = 5 of 10) failed to yield pregnancies after mating for 2 weeks (3-4 weeks post-CDT), again indicating YCT-529 could affect fertility.
Modification of dosing regimens of YCT-529 to achieve close to 100% inhibition of spermatogenesis
The observation that half of the animals treated with 10 mg/kg of YCT-529 for 2 weeks became infertile encouraged us to explore dosing regimens that might yield 100% inhibition of fertility while maintaining the same compound burden. We therefore compared the effects of the same dose of YCT-529 (10 mg/kg/day) for a longer period (4 weeks) versus an increased dose of 20 mg/kg/day for 2 weeks. For both dosing regimens (n = 20/dosing regimen), 5 mice were sacrificed at one day and 4 weeks post-CDT and fertility was assessed in the remaining males.
Assessment of the higher dosing regimen of YCT-529 (20 mg/kg/day for 2 weeks) revealed that 14 of 15 males failed to yield pregnancies after mating for 2 weeks (during 3-4 weeks post-CDT), close to 100% inhibition of fertility (Fig. 2g). Interestingly, most males remained fertile during 1-2 weeks post-CDT (Fig. 2g), likely because there is a 10-day minimum time for epididymal transit and storage in the cauda epididymis before ejaculation20. By 8 weeks post-CDT, 6 of 10 mice regained fertility. By 12 weeks, full fertility resumed in all males but one, which displayed a lower number of embryos than the standard litter size10,11. We concluded that the higher dose of YCT-529 efficiently induced infertility, followed by recovery post-CDT.
In support of this effect on fertility, at one day post-CDT, a drop in testicular weight and reduced sperm counts were observed (Supplementary Fig. 4a, b). Morphological abnormalities (n = 2) included the previously observed reduced numbers of epididymal sperm and germ cell sloughing, a failure of spermatid translocation (Fig. 2i), abnormal round spermatids (Fig. 2j), and multinucleated giant cells (Fig. 2k) in the tubules. Several (n = 3) males exhibited severe disruption of spermatogenesis, with 66% of tubules displaying either pachytene spermatocytes or round spermatids as the most advanced germ cells (Supplementary Fig. 5a, b), spermatids that failed to translocate (7.7%), and a lack of sperm in the corpus and caput epididymides (Supplementary Fig. 5c, d).
At 4 weeks post-CDT, both a drop in testicular weight and reduced sperm counts were still observed. At the morphological level, several males still exhibited a failure of spermatid release (Fig. 3a), an excess of elongated spermatids aligned at the tubular lumen (Fig. 3b), tubules almost completely lacking entire layers of specific germ cells (Fig. 3c–e) and reduced numbers of epididymal sperm. In two males, spermatogenesis began to recover, but characteristic phenotypes, in particular failure of sperm release, were still observed.
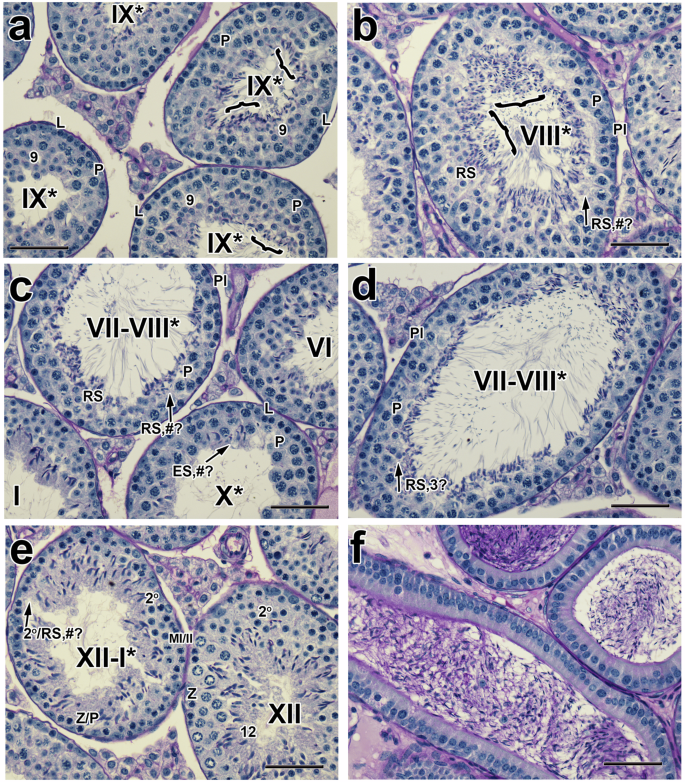
a–f Histological sections of testes and epididymides from mice treated with 20 mg/kg/day of YCT-529 for 2 weeks and terminated 4 weeks post-CDT. Several males still exhibited a failure of spermatid release (a, #1,16.7%; #2, 14.3% and #3, 8.5%), an excess of elongated spermatids (b, 5%, n = 1) aligned at the tubular lumen, along with tubules almost completely lacking entire layers of specific germ cells (c–e, #1, 36%; #2, 19.4%; #3, 25.8%) and reduced numbers of epididymal sperm. Pl, preleptotene spermatocytes; L, leptotene spermatocytes; P, pachytene spermatocytes; Z, zygotene spermatocytes; RS, round spermatids; ES, elongated spermatids; MI/MII, meiosis I and II; 2o, secondary spermatocytes. #?, the reduced number of specific cell types in a particular layer is indicated with pound symbol and question mark; RS, 3?, round spermatids, three only. Brackets in stage IX* tubules indicate retained spermatids. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the tubules. The drug-treated tubules are labeled with a Roman numeral followed by an asterisk (e.g., stage IX*). Scale bar in (a–f), 50 µm.
Males used for the mating studies were euthanized after recovery of fertility at 8–12 weeks post-CDT. They exhibited normal testicular weight and cauda epididymal sperm counts (53.90 × 106 ± 9.34 versus control 55.5 ×106 ± 8.18, n = 10). Testes contained 3–4 layers of spermatogenic cells, and the epididymides were full of sperm, indicating the recovery of spermatogenesis concomitant with restored fertility. Interestingly, normal testicular morphology was observed in one male that yielded fewer embryos.
We next assessed males treated with 10 mg/kg/day for 4 weeks (n = 20) and observed, interestingly, that infertility was induced more quickly post-CDT with this regimen. At one day post-CDT, 13 out of 15 drug-treated males failed to yield pregnancies after mating for 2 weeks (Fig. 2h versus Fig. 2g). The other two males yielded only one embryo each (Fig. 2h). At 4 weeks post-CDT, six of the 15 mice regained fertility (Fig. 2h), suggesting that some of the males could recover more quickly than those treated with the higher dose regimen (Fig. 2g). By 6 weeks post-CDT, 9 of 10 mice regained fertility, and by 10 weeks, fertility had resumed completely (Fig. 2h). The extended low dose regimen (10 mg/kg for 4 weeks) thus achieved close to 100% infertility and full recovery of fertility.
This regimen also yielded a greater drop in testicular weight (0.08 g ± 0.01 vs higher dose, 0.09 g ± 0.02) and sperm counts (5.42 ± 3.92 x 106 vs. higher dose regimen, 25.30 ± 14.37 x 106) one day post-CDT (Supplementary Fig. 4a, b versus Supplementary Fig. 6a, b). Histological evaluation revealed more severely disrupted testicular morphology than at the same time point in the higher-dose regimen, with tubules displaying either pachytene spermatocytes or round spermatids as the most advanced germ cells (Fig. 4a, n = 3). Vacuolar-like spaces in tubules were particularly prominent (Fig. 4c, d), and there was a lack of epididymal sperm in the corpus and caput epididymides (Fig. 4e, n = 4). If spermatids were present, they nonetheless failed to translocate (15.9%, n = 1), and there was almost a complete lack of sperm and the presence of sloughed germ cells in the corpus and caput epididymides (n = 1/5).
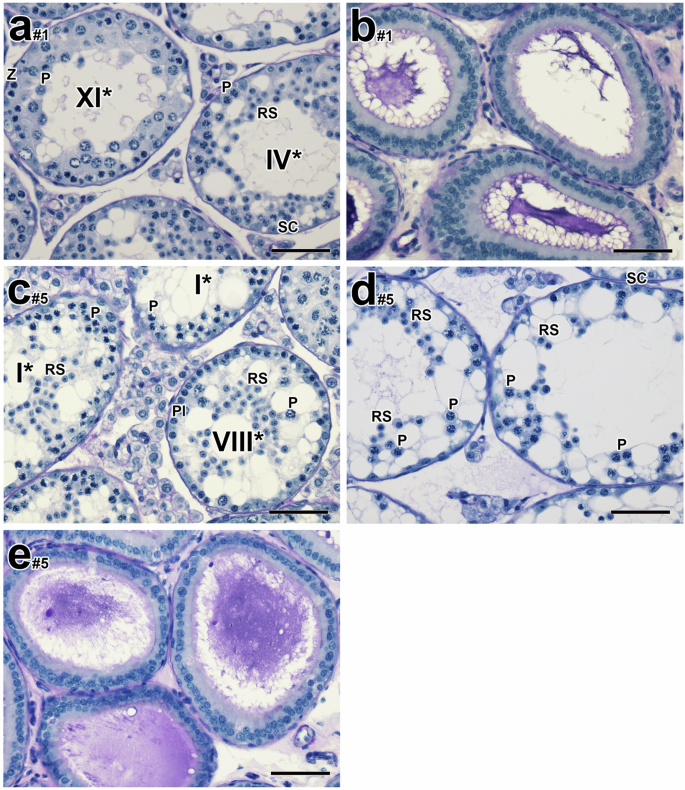
a–e Histological testicular and epididymal sections of male mice treated with 10 mg/kg/day of YCT-529 for 4 weeks and terminated one day post-CDT. Histological evaluation revealed more severely disrupted testicular morphology than at the same time point in the higher-dose regimen, with tubules displaying either pachytene spermatocytes or round spermatids as the most advanced germ cells (a, #1: 89.1%; #2: 96.3%; #3: 76.4%, n = 3). Pl, preleptotene spermatocytes; L, leptotene spermatocytes; P, pachytene spermatocytes; Z, zygotene spermatocytes; RS, round spermatids; SC, Sertoli cells. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the tubules. The drug-treated tubules are labeled with a Roman numeral followed by an asterisk (e.g. stage IX*). Scale bar in (a–e), 50 µm.
Four weeks post-CDT, a partial recovery in testicular weight (0.10 g ± 0.01) and a clear recovery in sperm counts (35.50 ± 12.95 x 106 vs the higher dose regimen, 9.79 ± 6.23 x 106, n = 5) were observed (Supplementary Fig. 4a, b versus Supplementary Fig. 6a, b). Recovery of spermatogenesis was observed morphologically in the majority of tubules (Fig. 5a, n = 5), with some remaining residual characteristic abnormalities, in particular failure of sperm release (Fig. 5b, n = 5). Other characteristic residual abnormalities included tubules with either pachytene spermatocytes or round spermatids as the most advanced germ cells (Fig. 5c, n = 5) and reduced numbers of epididymal sperm and some tubules almost completely lacking entire layers of specific germ cells (n = 2). Males from the mating studies were euthanized at 8–10 weeks post-CDT. Concomitant with their recovered fertility, they exhibited normal testicular weight, cauda epididymal sperm counts (57.90 ± 12.64 x 106 versus control 55.5 ± 8.18 x 106, n = 10), and normal testicular histology with tubules containing 3-4 layers of spermatogenic cells, and the epididymides were full of sperm (Fig. 5d), all indicating complete recovery of spermatogenesis.
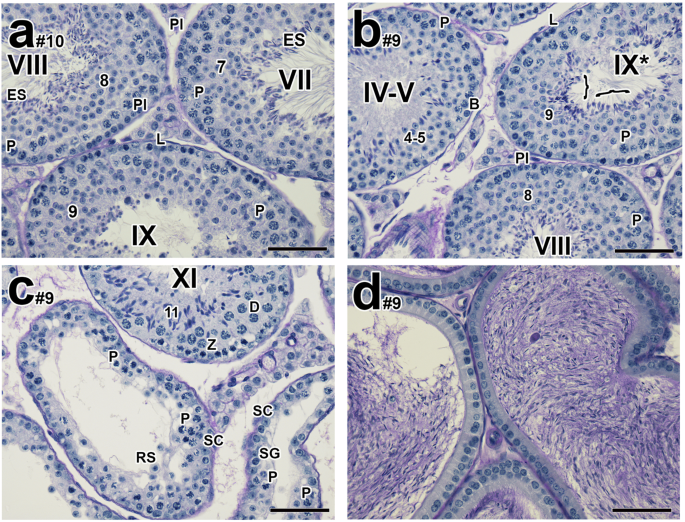
a–d Histological testicular and epididymal sections of male mice treated with 10 mg/kg/day of YCT-529 for 4 weeks and terminated 4 weeks post-CDT. A recovery of spermatogenesis was observed in the majority of tubules (a #1: 79.6%; #2: 92.5%; #3: 97.9%; #4: 85.8%; #5: 82.3%, n = 5) with some remaining residual characteristic abnormalities, in particular failure of sperm release (b #1: 5%; #2: 5.2%; #3: 2.1%; #4: 2.3%; #5: 1.8%, n = 5). Other characteristic residual abnormalities included tubules with either pachytene spermatocytes or round spermatids as the most advanced germ cells (, #1: 9.5%; #2: 0.9%; #3: 0%; #4: 11.9%; #5: 16.4%, n = 5) and reduced numbers of epididymal sperm and some tubules almost completely lacking entire layers of specific germ cells were observed (#1: 5.9%; #1: 2.3%, n = 2). Pl, preleptotene spermatocytes; L, leptotene spermatocytes; P, pachytene spermatocytes; Z, zygotene spermatocytes; D, diplotene spermatocytes; RS, round spermatids; ES, elongated spermatids; B, type B spermatogonia; SC, Sertoli cells; SG, spermatogonia. MI/MII, meiosis I and II. Bracket in stage IX* tubules indicates retained spermatids. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the tubules. The drug-treated tubules are labeled with a Roman numeral followed by an asterisk (e.g., stage IX*). Scale bar in a–d, 50 µm.
Assessment of the effects of YCT-529 in Cynomolgus macaques
Six sexually mature, non-naïve male Cynomolgus macaques (8–15 years, 6-15 kg) were used for PK and efficacy studies. The animal use protocol was approved by WuXi App Tec’s IACUC per the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guideline.
Bioavailability
The next logical step was to determine whether oral administration of YCT-529 could inhibit spermatogenesis in a higher mammalian species. We chose Cynomolgus macaques (Macaca fascicularis), a well-suited non-human primate (NHP) model in contraceptive research21. Initially, plasma levels were determined in 3 sexually mature males who received single oral doses of 1, 5, and 10 mg/kg of YCT-529. Supplementary Fig. 1b and Supplementary Table 1 show that YCT-529 became readily bioavailable in plasma, that there was no accumulation over time, that YCT-529 was substantially cleared within 48-96 hours, and that the exposure (Cmax and area under the curve [AUC]) to the tested doses was dose-proportional.
In two subsequent studies, blood was collected from Cohort 1 males (dosed at 0.5 mg/kg/day) and Cohort 2 (dosed at 5 mg/kg/day) on Days 1 and 28 to assess plasma PK profiles after repeated dosing. Supplementary Fig. 1c, d and Supplementary Table 1 show that YCT-529 became readily bioavailable and that there was no substantial accumulation over time. YCT-529 substantially cleared within 24 h. Twenty-four hours post-CDT (end of the dosing period), YCT-529 was detectable in plasma at concentrations of 298 ± 137 ng/mL (Cohort 1) and 694 ± 404 ng/mL (Cohort 2) but below the lower limit of quantification ( < 20 ng/mL) in seminal fluid samples of all animals (Supplementary Table 1).
We next asked whether oral administration of YCT-529 to Cynomolgus macaques resulted in a reduction in sperm counts. Sexually mature males were dosed with YCT-529 until sperm counts per ejaculate dropped below 200 x 106 cells (mean plus one standard deviation), as animals with sperm counts of 130 x 106 ± 70 cells per ejaculate are considered poor candidates for breeding programs22. The initial dose level of 0.5 mg/kg/day was chosen based on the dose (AUC) similar to that found in mice dosed at 10 mg/kg (Supplementary Fig. 1a and Supplementary Table 1). Cohort 1 (n = 3) was subjected to dosing and recovery regimens outlined in Fig. 6a. After dosing at 0.5 and 1.5 mg/kg/day (51 days in total), sperm counts of all 3 animals remained above 200 x 106 per ejaculate (Fig. 6a). After the dose was increased to 2.5 mg/kg, which is the dose equivalent to 10 mg/kg in mouse based on allometric scaling23, sperm counts dropped and remained below 200 x 106 per ejaculate during the remaining dosing period as of week 12 (animal #1; 32 days on 2.5 mg/kg/day), week 13 (animal #3; 39 days on 2.5 mg/kg/day), and week 21 (animal #2; Day 39 of the recovery period). Sperm counts per ejaculate in all animals reached or exceeded pre-dose baseline levels 78-148 days post-CDT. Cohort 2 (n = 3) was subjected to dosing and recovery regimens outlined in Fig. 6b. After dosing at 5 mg/kg/day for 14 days, sperm counts of animals #5 and #6 dropped and remained below 200 x 106 cells per ejaculate as of week 2, and as of week 5 in animal #4 (Fig. 6b). Sperm counts per ejaculate in all animals reached or exceeded pre-dose baseline levels 73-107 days post-CDT. Supplementary Fig. 7a, b shows sperm concentrations in millions per milliliter over time in Cohorts 1 and 2 as a standardized readout independent of ejaculate volume.
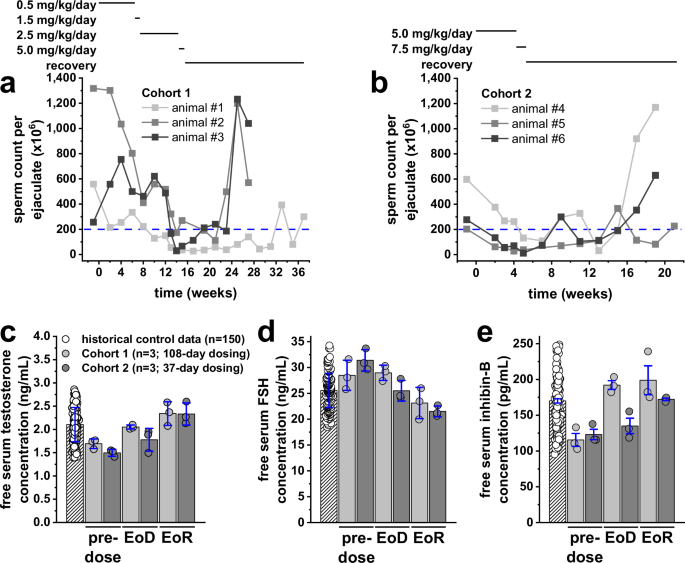
Three sexually mature male animals were dosed per cohort. Fresh semen samples were obtained via electroejaculation at indicated time points. Cohort 1 (n = 3) was dosed with YCT-529 at 0.5 mg/kg/day (Day 1-46) followed by 1.5 mg/kg/day (Day 47-51), 2.5 mg/kg/day (Day 52-101) and 5 mg/kg/day (Day 102-108); the recovery phase was from Day 109 to Day 257. Cohort 2 (n = 3) was dosed with YCT-529 at 5 mg/kg/day (Day 1–30), followed by 7.5 mg/kg/day (Day 31-37); the recovery phase was from Day 38 to Day 145. The blue dashed line in panels (a) and (b) indicates the upper limit of reported sperm counts of non-breeders22. Shown are the individual sperm counts per ejaculate (light grey, dark grey, and black lines) of Cohort 1 (a) and Cohort 2 (b) animals. Blood from all Cohort 1 and Cohort 2 animals was collected at pre-dose, 24 h post-CDT (end of the dosing period [EoD]), as well as 78 and 148 days (Cohort 1), and 93 and 107 days (Cohort 2) post-CDT (end of the recovery period [EoR]) to determine serum hormone levels with ELISA. Each sample was measured in duplicate. Shown are free serum testosterone (c), FSH (d) and inhibin-B (e) levels as individual historical control values (white circles) of 150 control animals and individual values of all Cohort 1 animals (light grey circles) and Cohort 2 animals (dark grey circles) at pre-dose, EoD and EoR. White striped, light grey, and dark grey bars represent mean values from all 150 historical control animals, all 3 Cohort 1 animals, and all 3 Cohort 2 animals, respectively, ± S.D. (in blue). No significant differences were noted as per one-way ANOVA analyses.
Correlation of changes in sperm counts with testicular histopathology
To correlate changes in sperm counts with impaired and resumed spermatogenesis at the histological level, testicular biopsies were obtained 24 h post-CDT (end of the dosing phase) and on the respective last day of the recovery period (Days 187 or 257 post-CDT in Cohort 1; Days 131 or 145 post-CDT in Cohort 2). In biopsies obtained 24 h post-CDT, vacuolization of seminiferous tubules and disorganization of the seminiferous epithelium were noted in all samples. Approximately half of the tubules were devoid of germ cells, while the other half contained germ cells, which appeared to be retained in the germinal epithelium or trapped in the vacuoles. Lesions noted in animals of Cohort 1 appeared to be less severe than those in Cohort 2 (Supplementary Fig. 7c, e). At the end of the recovery period, only slight degeneration of a few seminiferous tubules was noted, indicating that all animals of both cohorts were recovering (Supplementary Fig. 7d, f).
Hormonal homeostasis and clinical chemistry
To determine if YCT-529 resulted in a disturbance of hormonal homeostasis, free serum testosterone, FSH, and inhibin B levels were assessed before dosing (Day 1), 24 h post-CDT (end of dosing), and on the respective last day of recovery (Days 187 or 257 post-CDT in Cohort 1; Days 131 or 145 post-CDT in Cohort 2). Mean testosterone (Fig. 6c), FSH (Fig. 6d), and inhibin B (Fig. 6e) levels varied throughout the study between 1.50–2.48 ng/mL, 21.28–31.40 ng/mL and 115.44–198.84 pg/mL, respectively. Pre-dose values were within normal ranges, and respective levels at the end of the dosing and recovery periods were not different from Day -1 values in all animals.
Blood was collected before dosing, on Day 28, 24 h post-CDT, and on the respective last day of recovery to assess serum chemistry and hematology. All parameters (summarized in Supplementary Table 2) were within control ranges at all time points in all animals of Cohort 1 and Cohort 2.
Discussion
A potential male contraceptive that is administered orally would be the best-case scenario for ease of use and compliance. Data from a mouse PK study (Supplementary Fig. 1a) confirmed that YCT-529 had sufficient exposure to produce a pharmacological effect after a single oral dose of 10 mg/kg. The brain was examined in addition to the testes because both tissues exhibit a blood-tissue barrier24. Interestingly, levels in the testes (900 ng/g, 2 µM) appear almost equivalent to those found in the plasma, suggesting passive diffusion across the blood-testis barrier is occurring. Brain levels (343 ng/g, 0.7 µM) were less than half of plasma or testis levels. The observed delay in Tmax may be accounted for by the time it takes for molecules to pass through the blood-testis barrier by passive diffusion. Nevertheless, these concentrations of YCT-529 exceed its IC50 for RARα antagonism by 300-fold. Sufficiently high concentrations remain in the testes after 24 h (~200 ng/g or 0.4 μM) to exceed the IC50 by approximately 65-fold, suggesting that once-daily administration would be adequate to achieve and maintain a pharmacological effect.
Given the specificity of action of YCT-529 against RARα and its favorable in vitro and pharmacokinetic properties, we tested its in vivo efficacy in inhibiting the progression of spermatogenesis. We first turned to our well-characterized mouse model, based on years of dietary, pharmacological, and genetic studies that underscore the importance of retinoid signaling and RARα, particularly in maintaining male fertility. Indeed, our detailed analyses revealed that the RARα-selective drug YCT-529 elicited profound effects on spermatogenesis in a dose-dependent manner that ultimately resulted in the inhibition of fertility. Importantly, the cellularity of the effects was similar to those observed upon vitamin A deficiency, genetic ablation of Rara, and pharmacological inhibition of RAR antagonists10,11,25. Most importantly, for a compound of potential applicability to male contraception, the inhibition of fertility was reversible. That is, sperm numbers were restored, spermatogenesis resumed at the histological level, and pregnancies resulted without any observable side effects. Furthermore, we noted a more rapid recovery in the lower dose mice (10 mg/kg for 4 weeks) compared to the 20 mg/kg group treated for 2 weeks, similar to observations with BMS-18945311. It was interesting that we observed the acute disruptive effect of YCT-529 on sperm counts and ensuing fertility in mice treated with several dosing regimens before a full cycle of spermatogenesis (35 days) had lapsed. Indeed, this disruption was evidenced in the histological evaluation of the testes and epididymides, with spermatocytes and spermatids as the most advanced germ cells. Further, improper alignment of spermatids suggested that the spermatid-Sertoli cell interactions were disrupted. The immediacy of the effects likely reflects that YCT-529 affects later stages of the spermatogenic cycle and supports the efficacy of its function.
As the human application of this pharmacological approach is our ultimate goal, we next pursued studies in non-human primates (NHP). A 0.5 mg/kg/day dose of YCT-529 resulted in similar PK values as in the mouse but was insufficient to decrease NHP sperm counts below the set threshold in any of the 3 animals after 46-day repeat dosing. After increasing the dose to 2.5 mg/kg/day, sperm counts of all 3 animals decreased and remained below 200 million sperm cells per ejaculate after dosing for 2–6 weeks and reached or exceeded pre-dose baseline levels 78–148 days post-CDT, indicating full recovery. Similar timelines were noted in Cohort 2 (n = 3) animals dosed once daily at 5 mg/kg/day (twice the efficacious concentration of Cohort 1) for the first 30 days. Sperm counts of all 3 animals decreased and remained below 200 million sperm cells per ejaculate within 2–5 weeks of dosing and reached or exceeded pre-dose baseline levels 93–107 days post-CDT.
It was noted that sperm counts did not drop equally fast in the six animals studied. Nonsynchronous response times are not uncommon, especially with small cohorts. Such variations were also recently described in a clinical study with men to assess dimethandrolone undecanoate as an oral hormonal male contraceptive26. Changes in sperm count correlated with the histopathology of testicular biopsies that revealed vacuolization and disorganization of the germinal epithelium at the end of the dosing period. At the end of the recovery period, normal testicular morphology was observed in all 6 animals. Although the morphology of the testicular biopsies was insufficient to permit assessment of what stages of spermatogenesis were impaired, the overall appearance of the testicular tissue was similar to our current findings in mice dosed with YCT-529 and to previous findings from studies with BMS-189453 in mice10, rats, and rabbits9.
There were no significant changes in testosterone, FSH, and inhibin B hormone concentrations during or after 108- or 37-day repeat dosing. Respective levels at the end of the dosing and recovery periods were not different from Day 1. This supported the conclusion that the changes in sperm count were due to YCT-529 activity and not to hormone changes and agrees with findings from a mouse study with BMS-18945310.
No statistically significant changes in hematology parameters, including neutrophil granulocyte counts, were noted in any animal of Cohorts 1 or 2 compared to Day 1. RARα has been reported to be the major RAR to mediate neutrophil granulocyte maturation27. However, YCT-529 had no statistically significant changes in neutrophil counts, which could be because the concentration of YCT-529 in bone marrow was too low to antagonize RARα or that the expression of RARβ and/or RARγ is upregulated to compensate for the loss of RARα activity. All serum chemistry parameters of Cohorts 1 or 2 animals were in the normal range28.
Secondary drug transfer through seminal fluid can potentially result in local and/or systemic effects in sexual partners29. Our results show that concentrations in the seminal fluid were at least 8-fold lower than in plasma, indicating that in NHPs, YCT-529 is either secreted into the seminal fluid at small concentrations or not at all. Therefore, the possibility of seminal transfer of YCT-529 is low.
Conclusions
The present study shows that the sequential chemical, biochemical, pharmacological, and biological combination identified the new RARα-specific molecule. YCT-529 exploits the retinoid signaling pathway in the testis as a novel target for male contraception. Oral administration of YCT-529 inhibits fertility reversibly in the mouse model with disrupted spermatogenesis and with cellular specificity predicted for specific inhibition of the RARα pathway. Further, YCT-529 reversibly inhibits sperm production in an NHP model, with no detectable adverse side effects, laying the groundwork for evaluating YCT-529 in human clinical trials.



Responses