Temporal evolution of breast cancer brain metastases treatments and outcomes

Background
Breast cancer (BC) represents the most common cancer diagnosed in women worldwide and one of the leading cause of cancer death, with over 680 000 deaths estimated each year1,2. Brain metastases (BMs) represent a common complication of metastatic BC and one of the main causes of death among BC patients, with around 30 to 50% of patients with Human Epidermal Growth Factor Receptor 2- positive (HER2+) and triple-negative metastatic BC expected to develop central nervous system involvement (CNS) during the course of their disease3. Moreover, the incidence of BMs in metastatic BC patients is progressively increasing over time, mainly due to the use of more effective systemic treatment, better control of extracranial disease and longer survival of patients with metastatic BC and to the widespread use of more sensitive imaging techniques4.
Despite remaining an unmet medical need, over the last years treatment strategies for BCBMs have greatly evolved, both in terms of local and systemic treatments. Among locoregional treatments, stereotactic radiosurgery has progressively become one of the preferred treatment options, as it avoids the risk of neurocognitive decline associated with WBRT and can be repeated in case of further CNS progression5. Additionally, even if the brain has historically been considered a pharmacological sanctuary, several systemic agents showing activity on BMs have become available in recent years. Indeed, anti-HER2 therapies and other targeted agents have demonstrated intracranial activity and clinical efficacy even in BMs6,7,8.
Despite these important modifications in the treatment of BCBMs and the increasing availability of drugs with expected activity on BMs, the actual impact of these changes on long-term patient outcomes in a real-life setting has not been explored yet.
The aim of this study is to assess the temporal evolution of clinicopathological characteristics and treatments of patients with BCBMs as well as outcomes and prognostic factors over the last two decades in a multicentric real-world setting.
Results
Patients characteristics
Overall, 779 patients with BCBMs were identified (375 at Istituto Oncologico Veneto, 300 at Montpellier Cancer Institute and 104 at Center Antoine Lacassagne) and classified based on the year of BMs diagnosis: 241 in group A (2000-2007), 323 in group B (2008-2014) and 215 in group C (2015-2022) (Supplementary Table 1).
Main clinicopathological characteristics at BMs diagnosis are summarized in Table 1.
Median age at BMs diagnosis was 55 years (range 23-89), with a significant proportion of patients under 50 years in all three time groups and no significant difference observed between the three groups. As expected, most patients, across the three time groups, were diagnosed with invasive carcinoma of no special type and histological grade 3 tumors. However, the percentage of patients presenting histological grade 3 BCs (as opposed to grade 1-2 BCs) increased significantly over time (p = 0.018).
Another significant change was observed in subtype distribution, with a progressive increase over time of the proportion of patients with HR+ BCs, which rose from 49.8% in group A to 63.6% in group C (p = 0.012). This observation was confirmed in both HR+/HER2+ (17.1% to 24.9%) and HR+/HER2- (30.8% to 38.5%) subgroups.
The large majority of patients throughout the three time groups presented a relapse of a previously stage I-III BC; however, a progressive increase in the proportion of patients diagnosed with de novo metastatic disease was also observed (16.6% to 28.4%; p = 0.009), while the proportion of patients diagnosed with BMs at time of first metastatic BC diagnosis and which presented extracranial disease at time of BMs diagnosis remained relatively constant overtime (p = 0.523 and p = 0.652, respectively).
Interestingly, a significant improvement in the proportion of patients presenting a conserved performance status at time of first BMs diagnosis was observed (KPS 90-100 in 22.4% in group A and 33.5% in group C; p = 0.007). In addition, a not statistically significant numerical trend towards a higher proportion of patients diagnosed with single brain lesions in the more recent group (33.5% vs 20.3% in group A, p = 0.084) was observed.
Treatments administered after brain metastases diagnosis
Locoregional and systemic treatments received after BCBMs diagnosis are detailed in Table 2.
While no significant change in the proportion of patients undergoing neurosurgery was observed over the three time periods (p = 0.401), a significant increase in stereotactic radiotherapy use (p < 0.001) and a concomitant decrease in whole-brain radiotherapy (WBRT) use (p < 0.001) were reported. A significant association between KPS at time of first BM diagnosis and stereotactic radiotherapy use was observed, with patients with conserved performance status being more likely to receive stereotactic radiotherapy (p < 0.001), while no significant association between KPS and WBRT administration was found (p = 0.077).
Regarding systemic therapies, no significant change in the proportion of patients treated with chemotherapy was observed over time (p = 0.463), while a numerical increase in endocrine therapy use after BM was observed among HR+ BC patients (23.6%, 33.1%, 38.1% in group A, B, C, respectively; p = 0.056). More strikingly, a significantly higher proportion of patients with HER2+ BCs received at least one anti-HER2 therapy after BM diagnosis in more recent time periods as compared with earlier time periods (64.8%, 76.1%, 83.5% in group A, B, C, respectively; p = 0.011). In addition, patients treated more recently also generally received a higher number of HER2-directed therapy lines (p = 0.001).
Overall survival
At last follow-up, the large majority of the patients included in this study (91.7%, 714/779) had died. In the overall study cohort, no significant change in OS from first BM diagnosis was observed across the three time periods assessed (median OS 8.2 months, 8.7 months, 8.4 months in group A, B, C, respectively; p = 0.260, Fig. 1).
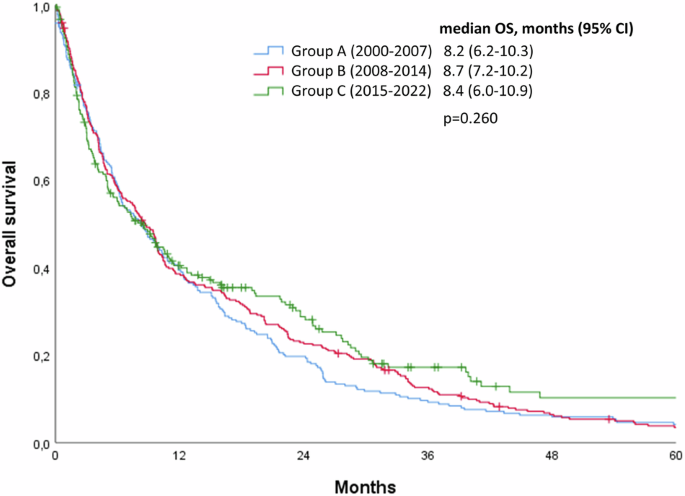
Kaplan-Meier curves showing the impact of year of diagnosis on overall survival from time of brain metastases diagnosis in the overall study cohort.
We then analyzed separately each BC subtype to assess whether changes in OS from first BM diagnosis might be observed in a specific subgroup. Indeed, a significant progressive improvement in OS from first BM diagnosis was selectively observed in patients with HR-/HER2+ BCs (median OS 8.7 months, 10.1 months, 23.7 months in group A, B, C, respectively; p = 0.002), while no significant change in OS from first BM diagnosis was observed in the other BC subtypes over the three time periods taken into analysis (Fig. 2). A significant OS improvement was observed in the whole HER2+ cohort (Supplementary Fig. 1), probably mainly guided by the improvement observed in the HR-/HER2+ subgroup.
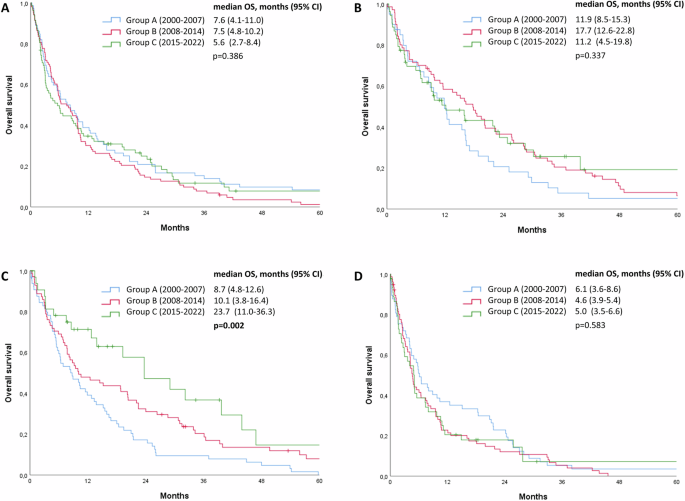
Kaplan-Meier curves showing the impact of year of diagnosis on overall survival from time of brain metastases diagnosis in HR+/HER2- breast cancers (A), HR+/HER2+ breast cancers (B), HR-/HER2+ breast cancers (C) and HR-/HER2- breast cancers (D).
Across all three time intervals, brain progression-encompassing both progression of existing pretreated lesions and the development of new lesions-remained the main cause of death in patients diagnosed with BMs (60.6%, 51.2% and 53.8% of patients in group A, B and C, respectively, p = 0.715). This was also confirmed when each BC subtype was analyzed separately (Supplementary Table 2).
Evolution of prognostic factors
Therefore, we assessed whether the prognostic impact of relevant clinical-pathological factors had changed over the different time periods evaluated. Univariate and multivariate Cox models assessing the association of each variable with OS from first BMs diagnosis are reported in Supplementary Tables 3,4,5. While some well-known factors, such as age, KPS and number of BMs, maintained their prognostic impact quite consistently throughout all the three time periods (Fig. 3), HER2-positivity showed no prognostic role in group A (p = 0.958) and emerged as a strong positive prognostic factor in groups B and C (p < 0.001 at multivariate analysis in both groups; Fig. 3, Supplementary Tables 2, 3). The recently proposed Updated Breast GPA score, which takes into account Performance Status, BC subtype, age, number of BMs, and extracranial disease status, showed a prognostic value across all three time intervals (p < 0.001) (Supplementary Fig. 2). However, a clearer separation of the curves was observed in most recent time intervals (B and C), potentially linked to the increase in the prognostic value of some components of this score (HER2-positivity and number of BMs) observed in more recent years.
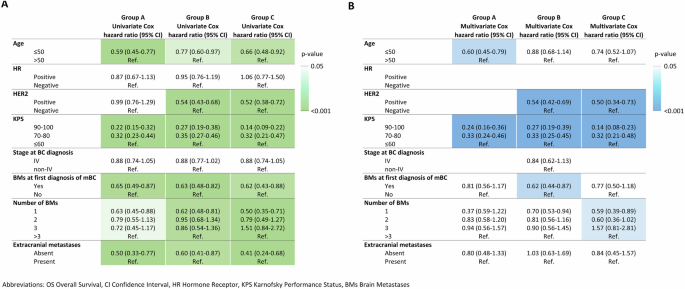
Heatmap summarizing the prognostic impact of each clinical-pathological variable across the three time periods by reporting in text the Hazard Ratio of the Cox Model, while the colour represents the level of significance reached by each variable in the univariate (A) and multivariate (B) Cox Model.
Discussion
Considering the clinical complexity of BMs and their increasing incidence, it is of great interest to assess changes in treatment strategies over the last decades and whether these have led to improvements in patient outcomes. This large, multicentric, real-world cohort allowed to accurately evaluate treatment evolution and potential changes in survival outcomes of patients with BCBMs over two decades.
Consistent with previous literature9,10, we observed in our cohort a relatively young median age at first BM diagnosis (with a significant proportion of patients younger than 50 years) and an enrichment in high-grade tumors which was maintained throughout the three time intervals. Interestingly, we observed a progressive increase in the relative proportion of patients with BCBMs presenting a HR+ BC, potentially reflecting an improvement in extracranial disease control and survival in these patients (for instance with the introduction of new endocrine based agents and CDK4/6 inhibitors, in addition to PARP inhibitors for the subgroup of patients with germline BRCA mutations), which might therefore be more exposed to the development of BMs. Indeed, among patients with HR+ BCs, CNS involvement generally represents a late complication in the natural history of the disease, and this could also explain the increase in time interval between first distant metastases diagnosis and diagnosis of BM which was observed in our study cohort in more recent time intervals9. On the other hand, this might also at least in part be related to an increase in early HR- (both HER2+ and HER2-) BC cure rates, thanks to therapeutic improvements observed in this setting over the last two decades. Indeed, we also observed an increase in the proportion of de novo metastatic BC patients, which is in line with other studies11, and may be also explained by improvements in early-stage BC screening policies and therapeutic strategies and by the widespread use of more sensitive imaging techniques. Overall, these observations highlight an evolution in the baseline clinicopathological characteristics of patients with BCBMs.
The clinical management of patients with BMs requires a multidisciplinary and personalized approach, with a multimodal integration of locoregional and systemic therapies5. Locoregional treatments have been progressively refined, with the development of less invasive approaches such as radiosurgery and stereotactic radiotherapy. As expected, we observed in our cohort that, while the use of neurosurgery has remained relatively constant over the years, there has been a clear shift in the type of radiotherapeutic technique used, with a progressive increase in the use of stereotactic radiotherapy and a decrease in the use of WBRT, resulting in an overall decrease in radiotherapy utilization. Indeed, WBRT is known to carry a higher risk of neurocognitive decline and is usually not repeatable. Growing awareness of these neurocognitive sequelae has led guidelines to progressively limit the use of WBRT to selected cases5. In contrast, the use of stereotactic radiotherapy, which represents fewer long-term sequelae, can be applied to multiple lesions and can be repeated in case of further intracranial progression, has progressively increased12. Moreover, in more recent years, the introduction of systemic agents with relevant intracranial activity has led guidelines to consider the potential delay of WBRT in selected patients with HER2+ BC BMs5. Consistent with the well-known improvements observed in systemic treatment of metastatic BC, including the introduction of novel agents with intracranial activity13, we also observed significant variations in systemic treatments received by patients with BCBMs. In fact, we observed a significant increase in the use of anti-HER2 therapies after BMs diagnosis in HER2 + BC patients, as well as an increase in the number of anti-HER2 therapy lines administered. This trend is consistent with the growing availability and efficacy of anti-HER2 agents over time, with drugs such as T-DM1 and pertuzumab becoming available between 2013 and 2015. Additionally, since extracranial metastases represent a significant competitive risk of death in patients with BCBMs, the increased availability of effective systemic treatments underscores the need to systemically consider these therapies for BCBMs patients.
Despite these significant changes in the clinical management of patients diagnosed with BCBMs, no significant OS benefit was observed in the overall study cohort. However, when assessing separately different BC subtypes, a dramatic improvement was observed in patients with HR-/HER2+ BC, which presented a significant increase in median OS from 8.7 months in group A to 23.6 months in group C. This result seems primarily attributable to the greater availability of anti-HER2 therapies showing intracranial activity in addition to improved extracranial activity. Indeed, intracranial progression accounted for nearly 60% of deaths in patients with HR-/HER2+ BCBMs. While it is challenging to distinguish between the intracranial and extracranial effects of anti-HER2 therapies, the relevance of their intracranial activity appears significant, alongside their established extracranial benefits. Our results are also consistent with a large analysis of a real-world cohort of 20446 patients with metastatic BC (not selected for BMs), which observed a significant improvement in OS selectively in the HER2+ subgroup11. Our study further expands these data including more recent years and demonstrating that such selective benefit is also confirmed, and clinically relevant, in patients with HER2+ BMs, especially in the subgroup with HR-/HER2+ BC.
Unexpectedly, we did not observe a similar improvement in OS in the HR+/HER2+ subgroup. This might potentially be related to the different kinetic of BM development observed in HR+ as compared to HR- BC, as HR-positivity is usually associated with a later BMs diagnosis and therefore potentially with more pretreated patients)9,14, and with the higher biological heterogeneity of this subgroup which is thus potentially less responsive to anti-HER2 agents15. Additionally, despite significant improvements in the systemic treatment of HR+/HER2- and triple negative BCs, no OS benefit was observed in these subtypes. This observation should however be interpreted with caution, as some of the major modifications in the systemic treatment HR+/HER2- and triple negative BCs (e.g. CDK4/6-inhibitors and immune checkpoint inhibitors) have only very recently become available in clinical practice (e.g.palbociclib starting from 2017 and immune checkpoint inhibitors starting from 2019 in France and Italy). Therefore, their benefits may not yet be evident in our study cohort and might become more apparent in the future. Additionally, the development of BMs typically occurs later in the clinical history of HR+/HER2- BC, often after several treatment options have already been fully exploited, therefore potentially limiting the impact of advances in systemic treatment in improving the prognosis of this subgroup of patients14. However, it will be of interest to evaluate, in future years, the potential impact that the expansion of effective treatment options (e.g. trastuzumab deruxtecan in HER2-low BC)16 might have on long-term outcomes of patients diagnosed with BMs related to HR+/HER2- and triple negative BC.
Given the clinical complexity and the availability of treatments with different aggressiveness, adequate prognostic stratification of patients with BMs remains of crucial importance17,18. Despite the rapid evolution of the therapeutic landscape in BC, most prognostic scores have been developed in relatively old cohorts17,19. Our temporal analysis allowed to examine the evolution of prognostic factors, confirming some validated prognostic factors in patients with BMs (such as KPS) as well as shedding light on the emergence of new factors over time. The positive prognostic value acquired by HER2-positivity further confirms the impact that anti-HER2 treatments have had in this setting. This also highlights the importance of reassessing prognostic score as treatments landscape evolves18.
This study has some limitations. Its retrospective nature did not allow complete data collection for certain patients. For instance, the lack of specific data on the imaging modalities (e.g., CT or MRI) used to diagnose brain metastases do not allow to evaluate whether this might have potentially influenced detection rates (including the detection rate of leptomeningeal disease) and treatment decisions, especially during the first few years evaluated in our study. Moreover, the low numerosity of specific subgroups, such as patients without extracranial disease, may at least partially explain the lack of significance observed in multivariate analysis. Furthermore, the limited number of centers included in the study and the expected differences across the three centers in the number of patients included in the study in different time periods might potentially limit the generalizability of some of our observations, as these might also be linked to center-specific factors.
Additionally, as the diagnostic and therapeutic landscape of early and metastatic BC significantly changed over the two decades assessed by this study, the risk for a potential bias due to diagnostic anticipation and increased proportion of de novo metastatic patients in more recent time periods should be acknowledged. However, a significant OS improvement was only observed in a specific subgroup of BC patients, rather than being evident across all groups, which might mitigate this potential concern. Finally, in the future, the potential increase in proactive brain imaging for BMs in some subgroups of patients with metastatic BC could further modify the clinical and therapeutic scenario.
Nevertheless, this study has several strengths. It analyzes a large population of patients with BCBMs, covering a broad time span of over 20 years. Moreover, the comprehensive collection of clinical and pathological data enabled an integrated assessment of therapeutic advancements, clinical outcomes and prognostic factors in this setting.
In conclusion, in the last two decades, locoregional and systemic therapy of patients with BCBMs has significantly evolved. However, in this large multicentric real-world cohort, a significant OS improvement was only observed in patients with HR-/HER2+ BCBMs, probably due to the increased availability of anti-HER2 therapies with intracranial activity. In this evolving scenario, it is crucial to reassess prognostic factors, also considering the potential impact that the implementation of proactive brain imaging for HER2-positive and triple negative metastatic BCs might have in future years.
Methods
Patients
We conducted a retrospective study on consecutive patients newly diagnosed with BC-related BMs between 2000 and 2022 at three institutions: Istituto Oncologico Veneto – Padova (Italy), Montpellier Cancer Institute – Montpellier (France) and Center Antoine Lacassagne – Nice (France).
Inclusion criteria were: histologically proven BC, age ≥18 years at time of BC diagnosis, intraparenchymal BMs radiologically confirmed using contrast-enhanced brain computed tomography scan and/or magnetic resonance imaging of the brain. Patients diagnosed with isolated leptomeningeal involvement, in the absence of intraparenchymal BMs, were excluded, while patients with intraparenchymal BMs who were also diagnosed with leptomeningeal disease at time or after BMs diagnosis were included in the present study.
Patients were divided in 3 groups according to year of BMs diagnosis: 2000-2007 (group A), 2008-2014 (group B) and 2015-2022 (group C). The three time periods were identified based on changes in systemic therapeutic agent availability in Italy and France (such as pertuzumab and T-DM1) and on distribution of patients by year of diagnosis.
Demographic, clinicopathologic and treatment data were retrospectively collected from medical charts in a dedicated database. Hormone receptor (HR) expression was determined by immunohistochemistry on primary tumor; positivity was defined as immunohistochemistry staining in at least 1% of tumor cells according to ASCO-CAP guidelines. HER2 positivity (HER2+) was defined according to ASCO-CAP guidelines (immunohistochemistry score 3+ or immunohistochemistry score 2+ in presence of amplification of the HER2 gene by in situ hybridization), while HER2 negativity (HER2-) was defined, according to ASCO-CAP guidelines, as an immunohistochemistry score 0 or 1+ or immunohistochemistry score 2+ in absence of amplification of the HER2 gene by in situ hybridization20. WBRT was defined as external beam radiation treatment in which the entire brain is uniformly irradiated, typically at a dose of 30 Gy delivered in 10 fractions. Stereotactic radiotherapy was defined as a high-precision radiotherapy technique delivering 1–5 fractions of high-dose radiation, with a total dose typically ranging from 15 to 33 Gy (higher total doses typically administered in hypofractionated regimes), to small, well-defined targets. Updated breast GPA score was calculated according to the original publication21.
This study was reviewed and approved by the involved Institutional Review Boards and Ethics Committees. If necessary according to local regulation, written informed consent was obtained from participants. The study was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
Descriptive statistics were performed for patients’ demographics and clinical characteristics. Chi-squared (χ2) and Kruskal-Wallis tests were used to study association between variables as appropriate.
Distant metastases free survival was defined as time from BC diagnosis to distant metastases diagnosis. BM free survival was defined as time from first distant metastases diagnosis to BM diagnosis. Overall survival from BM diagnosis (OS) was defined as time from BMs diagnosis to death from any cause. Patients alive without event at the cut-off date of this analysis (1st November 2023) were censored at the date of last follow-up. Distant metastases free survival, BM free survival and OS were estimated using the Kaplan-Meier method and reported with its 95% confidence intervals (95% CIs). Log-rank test was used to compare OS between groups. Univariate and multivariate Cox regression modeling for proportional hazards was used to calculate hazard ratios and their 95% CI.
All reported p values were two-sided and significance level was set at 5% (p < 0.05).
Statistical analysis was performed using IBM SPSS (version 26).


Responses