The biomechanics of the vagina: a complete review of incomplete data

Introduction
The vagina is a fibromuscular tubular organ that connects the outside of the body to the cervix. It has four distinct structural layers: the epithelium, subepithelium, muscularis, and adventitia (Fig. 1). Each layer has a unique microstructure and serves a specific biological function. The epithelium, comprised of stratified squamous epithelial cells, defends against infection and undergoes periodic changes as part of the reproductive cycle. The subepithelium, also known as the lamina propria, houses abundant blood vessels. Its fibrillar extracellular matrix components, such as elastin and collagen, contribute to the structural integrity and strength of the organ. The muscularis, comprised of smooth muscle, acts as the primary contributor to vaginal contractility. Finally, the loose connective tissue of the adventitia, the outer layer of the vagina, provides additional strength to the organ connecting it to the adjacent bladder and rectum1. The proximal vagina (closer to the cervix) is formed from the paramesonephric ducts during congenital development, while the distal vagina (closer to the introitus) is formed from the mesonephric ducts2.
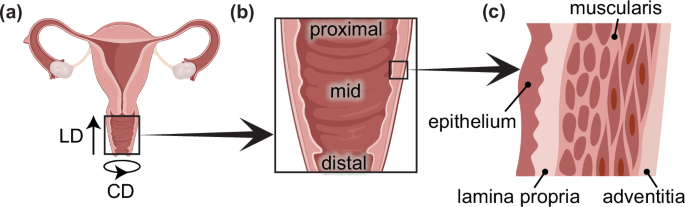
a Reproductive tract showing the longitudinal direction (LD) and circumferential direction (CD) of the vagina. b Proximal (closer to the cervix), mid, and distal (closer to the introitus) regions of the vagina. c Four distinct layers of the vagina.
The heterogeneous structure of the vagina dictates its unique mechanical attributes, which are crucial to the organ fulfilling its multiple physiological functions. Given its position within the pelvis, the vagina, in conjunction with pelvic connective tissues and skeletal muscles, provides direct mechanical support to pelvic organs, including the bladder and uterus3,4. It does so continuously while withstanding various stresses and deformations placed upon it by the surrounding organs and biological events. Most impressively, after undergoing extraordinary remodeling throughout pregnancy, the vagina accommodates the passage of the baby, stretching from its reference diameter of ~2.5 cm up to a diameter of 10 cm and withstanding large amounts of mechanical stress5.
Despite the extensive remodeling of the vagina and other pelvic organs throughout pregnancy, vaginal delivery can still cause maternal injury. Nearly 80% of all vaginal deliveries result in some degree of trauma to the vagina and surrounding tissues6,7. In addition to short-term morbidities, maternal birth injuries can lead to long-term physical and psychological sequelae8,9. Moreover, close to 30% of women who have undergone vaginal delivery will develop at least one pelvic floor disorder later in life, a rate which is nearly threefold higher than the 11% prevalence in women who have never been pregnant10. Many epidemiologic studies suggest that damage to the vagina and other pelvic supportive structures is a causative factor in the pathogenesis of pelvic organ prolapse and other pelvic floor disorders.
For the past two decades, several researchers have been working on characterizing the biomechanical properties of vaginal tissue recognizing the strong relationship between such properties and its biological function (Fig. 2). Knowledge of these properties can provide measurable and objective metrics on the impact of life events (e.g., pregnancy, childbirth, and menopause) and health conditions (e.g., prolapse) on vaginal tissue, yielding insights into the clinical care of women undergoing treatments for health issues such as vaginal birth injury and prolapse. Moreover, the characterization of the biomechanical properties of the vagina can guide the development of new biomedical approaches to address unmet clinical needs in women’s health11.
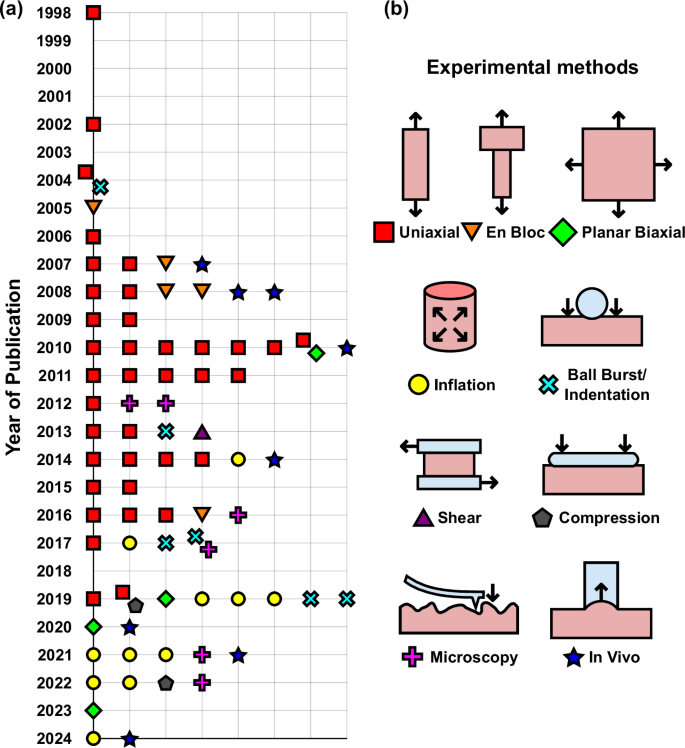
a Published experimental studies on mechanical testing of vaginal tissue characterizing passive (non-contractile) properties per year (refer to Table 1). Different colors and shapes indicate the different testing methods used, as indicated in b. Two symbols in the same location represent studies that used two mechanical characterization methods.
There are several experimental methods for determining the vagina’s biomechanical properties, each offering its own set of advantages and challenges. While in vivo techniques are the most physiologically relevant, the related ethical and technical constraints leave crucial gaps in knowledge that ex vivo methods prove invaluable in filling. Similarly, ethical and practical limits to human testing make using animal models invaluable12. The use of preclinical models enables researchers to study independent and combinatorial effects of age, pregnancy, parity, maternal birth injury, menopause, and prolapse on the mechanical properties of the vagina. Conversely, the interrelation of these factors, such as a correlation between age and menopause, often cannot be avoided in human testing13.
Vaginal morphology can differ significantly between individuals14, which undoubtedly contributes to the wide variations in mechanical behavior that have been reported in the literature. These discrepancies are also likely compounded by differences in the methods and protocols used for the mechanical characterization of vaginal tissue across different research groups. There is an unmet need to synthesize existing knowledge and identify common trends and conflicting results in biomechanics that need resolution. While there have been recent reviews on the active contractile properties of vaginal tissue15, and a more recent review on the effect of menopause on vaginal mechanics16, there has been no comprehensive review of the vaginal mechanical properties, and the impact of significant events on these properties.
The field of vaginal biomechanics has historically been under-researched despite its profound implications for women’s quality of life and, as such, it requires sustained and focused efforts to drive advancements. This in-depth review will critically evaluate the current literature from its inception, tracing the development of different methodologies over time. Despite the limited data, this review will identify consensus and highlight controversy about the effects of life events and health conditions affecting the biomechanics of this vital organ throughout women’s lives. The authors hope this synthesis will be a helpful resource for new researchers in biomechanics entering the emerging field of women’s health. Thus, the ultimate goal of this review is to address and close persistent clinically relevant knowledge gaps in women’s health by offering new directions for future studies on vaginal tissue biomechanics.
Mechanical testing methods
The first mechanical tests of the vagina were performed on tissue collected from rabbits in the 1950s17,18,19 (Fig. 3), after which no studies were published for several decades. Figure 2 depicts the published studies focusing on the biomechanical properties of the vagina with the year of publication since 1998 (excluding studies on the active, or contractile, properties presented in a review by Huntington et al.15). The authors for these studies with the corresponding experimental methods and animal models are reported in Table 1. Ex vivo uniaxial tensile testing and inflation testing are the techniques that have been used most frequently to characterize vaginal mechanics20, with a smaller number of studies using alternative methodologies such as planar biaxial testing, ball burst testing, and in vivo methods, as can be seen in Fig. 2.
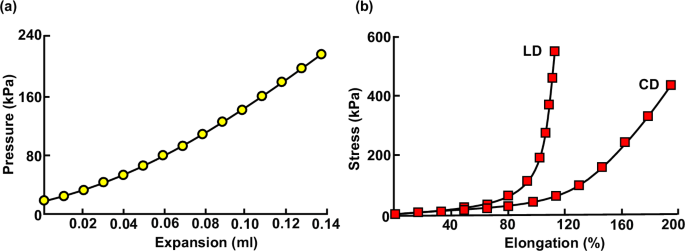
a Representative pressure versus expansion data adapted from Oda17 and b representative stress versus elongation data in the LD and CD adapted from Ohara18.
In uniaxial tensile testing, strips or rings of vaginal tissue are pulled in a single direction, the longitudinal direction (LD) or circumferential direction (CD) (Fig. 1a), to collect stress and strain data. These tests serve to characterize the mechanical response of vaginal tissue along one direction without considering axial coupling introduced by more physiologically relevant multiaxial loading. The most common form of uniaxial tensile testing is quasi-static loading, in which the tissue is pulled at a constant displacement rate, generally to failure or to a set maximum displacement or stress21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49. Such tests can be used to measure several mechanical properties, including tangent modulus (or, the slope of the linear portion of the stress-strain curve), strength (or, the maximum stress withstood before rupture), and distensibility (or, the maximum strain withstood before rupture). Modified quasi-static testing protocols have been employed to measure stress relaxation50 (or, the decrease in stress over time at a constant fixed strain), and hysteresis (or, softening of the tissue which results from cyclic loading)51. In a few uniaxial testing studies, alternative protocols have been adopted that involve incremental loading with intervals between successive steps in displacement52,53,54 or by superimposing small amplitude sinusoidal vibrations over slow quasi-static displacement rates55,56,57.
Uniaxial tensile en bloc tests, in which the vagina and the attached supportive tissues are excised and pulled together in the LD, have also been carried out. Quasi-static loading to failure in en bloc uniaxial tensile tests has been used to obtain load-displacement and stiffness data for the entire pelvic floor supportive complex58,59,60,61,62. While en bloc testing provides valuable information on the structural properties of the vagina and supportive tissues as a whole, it is not as well suited as other ex vivo mechanical testing methods for characterizing the mechanical behavior of vaginal tissue alone.
Planar biaxial tensile testing, or planar biaxial testing for short, performed by stretching square samples of vaginal tissue in tension simultaneously along the LD and CD, is the ideal testing method for characterizing the mechanical behavior of vaginal tissue along the LD and CD simultaneously. Since the deformation in the two directions is controlled independently, this testing technique can determine the anisotropy (or, directional-dependent mechanical properties) of the tissue better than other mechanical testing methods. For example, planar biaxial testing has been used to investigate the direction-specific differences in the tangent modulus of vaginal tissue under quasi-static biaxial loading63, as well as quantify the stress relaxation along the LD and CD under equibiaxial displacements31,64. Planar biaxial testing is also well suited for investigating the tear behavior in vaginal tissue65.
Ex vivo inflation testing, in which fluid is infused into the vagina to apply pressure, is the most popular alternative to uniaxial tensile testing. This testing method, similar to planar biaxial testing, enables the application of loads in multiple anatomical directions simultaneously while keeping the vaginal tissue in its native tubular configuration. The majority of ex vivo inflation studies have utilized inflation-extension protocols, in which the axial length of the vagina is controlled throughout testing while the vagina is pressurized66,67,68,69,70,71. A few studies have instead used free-extension inflation, during which the tissue can freely extend along the LD while pressurized72,73. Both protocols have been used to characterize the tissue’s response to inflation at a constant infusion rate and measure the tissue’s tangent modulus or rupture strength. Recently, free-extension inflation tests have been used to characterize creep (or, progressive increase in strain over time at a constant load) in vaginal tissue74,75. Tear propagation in vaginal tissue has also been analyzed using extension-inflation via a coaxial custom-made latex tube76.
In ball burst testing, circular taut specimens of vaginal tissue are held planar while a ball presses transverse to the specimens’ surfaces in the radial direction of the vagina. This testing method induces a multiaxial load simulating the complex stresses the tissue experiences in vivo. While ball burst studies do not directly provide information on the material strength and tangent modulus of vaginal tissue, they have been used to compute the force the tissue can withstand before rupture, and the relative stiffness of tissue at both low and high applied loads21,77,78,79,80. A similar testing method, ball indentation, involves indenting the center of a planar circular section of vaginal tissue with a weighted ball. This approach has been applied to measure the elastic modulus and creep properties of the vagina81.
Some other experimental techniques have been less commonly employed in the study of vaginal tissue’s biomechanics. Single-lap shear tests have been conducted by adhering the inner and outer walls of the vagina to plates that are then pulled apart. Cyclic single-lap shear testing has been utilized to analyze the rheological (or, mixed elastic and viscoelastic) properties of vaginal tissue82. More recently, optical coherence elastography, a form of elastography that measures deformations resulting from compressive forces applied through optical coherence tomography, was employed to characterize the elastic modulus of vaginal tissue ex vivo48,83.
While the methods mentioned above have characterized the biomechanics of the vagina at the macroscopic level, microscopy-based mechanical testing methods are capable of analyzing the mechanical contributions of the individual tissue’s microstructural components. Atomic force microscopy, the most commonly used form of microscopy-based testing method, has been used to quantify the elastic modulus of the vaginal tissue84, as well as the elastic modulus of the vagina’s collagen81,85,86,87 and muscle content85. Scanning haptic microscopy has also been adopted to assess the elastic modulus of vaginal tissue88. Both these methods involve the use of a probe that scans the surface of the vagina, measuring its topography and mechanical properties.
In vivo methods have also been developed for mechanically characterizing the vagina, offering clinically relevant data by loading the tissue in its native environment. However, these methods only provide gross measurements of biomechanical properties. One of the early in vivo investigations of vaginal tissue distensibility has been conducted using inflation with a catheter balloon and pressure transducer89. While this method has not since been used to characterize vaginal mechanics fully, balloon catheterization has been used for estimating the in vivo intravaginal pressures69,70,74, providing a reference configuration for ex vivo inflation experiments. A more common approach for in vivo experimentation involves using cutometer-like skin probes, which measure the vacuum pressure necessary to lift an exposed tissue area to a certain distance90,91,92,93. Vaginal manipulator devices have been developed and used for measuring applied forces during insertion of the devices in the vagina94. When utilizing the force data in conjunction with measurements of the movement of such devices, this technique is capable of providing some indirect measurements of the biomechanical behavior of the vaginal tissue in vivo, similar to cutometer-like probes. Recently, elastography, a technique in which relatively small stresses are applied to determine the elasticity of a material at low strains, has seen use to indirectly estimate the biomechanics of the vagina in vivo. There are multiple ways of loading the tissue during elastography, including the application of shear-waves95 or quasi-static compression96.
Impact of physiological and pathological events on the biomechanics of the vagina
While the mechanical properties of vaginal tissue can vary across individuals and species, some mechanical characteristics are remarkably consistent and have been noted by nearly every study of vaginal tissue. For example, the stress-strain relationship of the tissue is characterized by an initial toe region, in which the stress increases non-linearly with strain (Fig. 3b). Several studies have noted that after a certain strain, the tissue softens before rupture, likely due to damage as the tissue approaches failure at high strains25.
Because of the tissue’s nonlinear elastic response, measurements of the elastic modulus, the slope of the stress-strain curve at a specified strain, are commonly used for quantifying the tissue’s stiffness. These measurements provide metrics for assessing changes in vaginal tissue that are induced by natural events (e.g., pregnancy), clinical conditions (e.g., prolapse), surgical procedures (e.g., ovariectomy), or treatment (e.g., mesh implantation) across published studies. Figure 4 presents the reported moduli of vaginal tissue, categorized by testing method, animal model, and experimental group. In cases where studies reported different elastic moduli (e.g., moduli at different strain levels or anatomical directions), all such values are included.
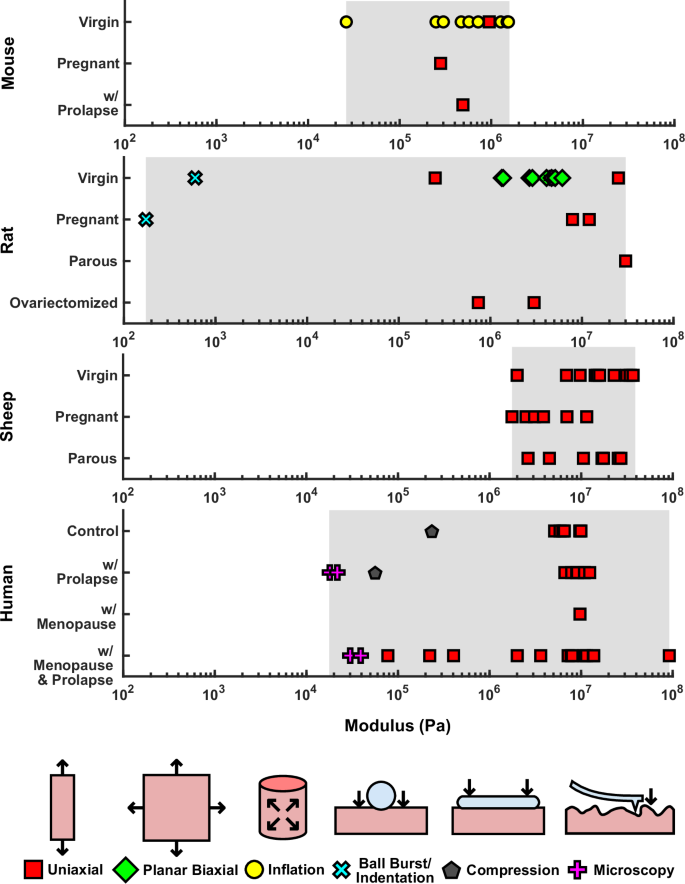
Reported values are categorized by animal models and human subjects and their conditions. Different shapes indicate various testing methods used, as indicated in the figure.
Several studies have noted significant anisotropy in the vagina, with differences in the stiffness of the tissue in the LD and the CD30,31,38,51,63. More often than not, in such studies, vaginal tissue in the LD was reported to be stiffer than in the CD, and it has been reported that anisotropy within the vagina is primarily a result of the orientation of its microstructural components, specifically of collagen fibers within the tissue. These observations of anisotropy are not universal. Some studies have noted no significant differences in stiffness between the LD and the CD36,65, and these differences may only become apparent at higher stresses or strains past the toe region65.
Vaginal tissue is viscoelastic, having time-dependent mechanical properties that are demonstrated by phenomena such as hysteresis, stress relaxation, and creep. The tissue exhibits hysteresis, with different mechanical responses to cyclic loading and unloading. While several studies have noted this behavior during preconditioning in both human vaginal tissue24,37 and animal vaginal tissue41,42, only Peña et al.51 have studied this phenomenon closely, characterizing Mullins-like effect in human vaginal tissue via tensile cyclic loading in the LD and CD.
The viscoelastic phenomenon of stress relaxation has been studied relatively more in vaginal tissue than the Mullins-type softening behavior. In their uniaxial study of prolapsed vaginal tissue from humans, Peña et al. observed near 50% decreases in stress in the LD after 15 min held at fixed stretch values of 1.3 and 1.450. Planar biaxial testing has been used to characterize the stress relaxation of vaginal tissue from rats and swine by Jing31 and Pack et al.64, respectively. Under equibiaxial displacement, Jing31 observed similar stress relaxation between the LD and CD, while Pack et al.64 observed higher stress relaxation in the LD than in the CD.
The creep behavior of vaginal tissue has been recently described in rodent models. Weli et al.81 were the first to measure creep, using indentation testing of rat vaginal tissue. Weli et al. did not characterize the creep behavior over time, measuring only the final displacement of the tissue after 24 h under indentation loading. Clark-Patterson et al.74 later characterized these time-dependent behaviors in the CD under extension-inflation testing of the murine vagina. In the wild-type mouse, increases in pressure resulted in greater amounts of vaginal creep, while variations in the fixed axial length of the vaginal canal had no significant effects on the tissue’s creep behaviors. Clark-Patterson et al. conducted consecutive creep tests, allowing for recovery between repeated creep tests. This is in contrast with a recent study by Dubik et al.75 evaluating the response of the rat vagina to multiple creep tests under free-extension inflation, without recovery between consecutive creep tests at progressively increasing intraluminal pressures. The creep behavior was observed to change significantly from the first creep test to the following creep tests, indicating that the mechanical behavior of vaginal tissue depends strongly on the full loading history of the tissue.
In the following sections, a detailed description of how life events and health conditions impact the mechanical properties of vaginal tissue is presented, with a few examples of mechanics-based evaluation of treatment strategies for pathological conditions. Since the vagina undergoes incredible remodeling to accommodate the passage of a fetus during vaginal delivery in humans97 and in other animal species98, its adaptations during the process of vaginal delivery have short-term and long-term consequences on the mechanical integrity of the vagina. Alterations in the mechanical properties of the vagina have also been linked with the incidence of pelvic organ prolapse99,100, characterized by the descent of the vagina and other pelvic organs from their normal anatomical position. Finally, systemic hormonal changes associated with menopause and aging also induce biomechanical changes in the vagina101.
Pregnancy and birth injury
Since experimental studies on the impact of pregnancy on vaginal mechanical properties cannot be conducted in pregnant women due to ethical and technical constraints, animal models have been used. Across every applied animal and testing modality, the effects of pregnancy on the mechanics of vaginal tissue are demonstrably consistent (Fig. 5). In uniaxial tests using incremental loading of murine vaginal tissue in the CD, Rahn et al.52 observed significant reductions in the stiffness and strength of vaginal tissue with pregnancy. At the same time, distensibility more than doubled in the pregnant tissue compared to virgin tissue. These findings were mirrored in vivo by Alperin et al.89, who found that pregnant rats accommodated a significantly greater increase in volume during balloon catheterization of the vagina than virgin rats, without reaching the same intravaginal pressures observed in virgin rats. In their uniaxial tensile tests of rat vaginal tissue collected in the CD, Jing31 also observed that pregnant tissue withstood greater ultimate strains than virgin tissue. Jing et al. also reported that the pregnant vagina had greater tensile strength than the nonpregnant vagina, making it the only study that has reported an increase in the strength of pregnant tissue compared to virgin tissue.
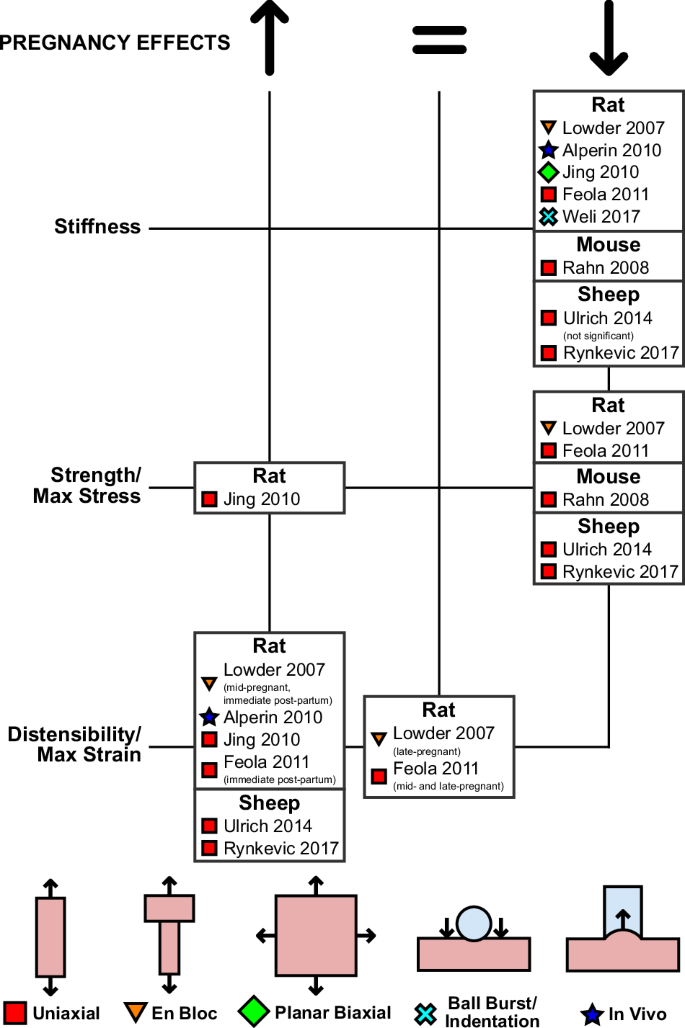
Only the first authors of the studies are reported, with the full list of authors provided in the list of references.
Effects of pregnancy on different anatomical regions of the vagina have been analyzed using uniaxial tensile testing in ewes. In both the ventral and dorsal regions of the vagina, Ulrich et al.42 observed large reductions in the tangent modulus and maximum stress and large increases in distensibility of the vagina with pregnancy. However, the changes in stiffness and strength were not statistically significant in specimens collected from the dorsal vagina, while the changes in stiffness and distensibility were not statistically significant in specimens collected from the ventral vagina. Rynkevic et al.47 compared the mechanical properties of the proximal and distal regions of the vagina before and during pregnancy. They observed a decrease in elastic moduli at high and low stresses, a decrease in the maximum stress, and an increase in maximum strain of both regions of the vagina with pregnancy. These differences were all statistically significant, with the exception of their comparisons of the elastic modulus at low stress, which were only significant in the proximal region, and not in the distal region of the vagina.
Whereas the aforementioned studies have only compared between virgin animals and late-pregnant animals, two studies have measured the alteration to the mechanics of vaginal tissue at both mid- and late-pregnancy, as well as immediately postpartum, in the rat model. Using en bloc uniaxial tensile testing, Lowder et al.59 observed the linear stiffness and ultimate load of the vagina with the supportive tissues decreased significantly in both mid- and late-pregnant rats compared to virgin animals. These changes persisted immediately after both vaginal delivery and cesarean delivery. The maximal distension of the tissue complex was also affected by pregnancy: tissues collected from mid-pregnant animals and immediate postpartum animals (after both vaginal and cesarean deliveries) reached significantly greater ultimate displacements than virgin tissue, but there was no significant difference between the maximum distension of tissues collected from virgin and late-pregnant rats. Using uniaxial tensile testing, Feola et al.34 observed the tangent modulus and strength of vaginal tissue in the LD decreased significantly at both mid- and late-pregnancy. These changes were progressive, as vaginal tissue collected from late-pregnant animals was weaker and more compliant than tissue from mid-pregnant animals. Furthermore, the rat vagina immediately following vaginal delivery was significantly weaker and more compliant than the virgin vagina. Feola et al. also compared the distensibility of the virgin, pregnant, and immediately postpartum vagina, finding that only the postpartum vagina could withstand significantly more strain than the virgin vagina.
Pregnancy has also been shown to have a considerable effect on the viscoelasticity of vaginal tissue. Jing31 utilized the planar biaxial testing method to characterize the stress relaxation of vaginal tissue collected from pregnant and nonpregnant rats. Vaginal tissue collected from both groups was stiffer along the LD than the CD, and pregnant tissue was more compliant than nonpregnant tissue. Furthermore, pregnant tissue exhibited greater stress relaxation in both the LD and CD than nonpregnant tissue, with similar stress relaxation between the two directions in both groups. Weli et al.81 later provided the first characterization of creep in vaginal tissue using ball indentation testing. They observed that vaginal tissue collected from pregnant rats experienced nearly fivefold greater deformation during creep testing than tissue from virgin rats, and had a 200% lower apparent elastic modulus. These authors associated these findings with changes in the microstructural components. Using atomic force microscopy, Weli et al. measured the stiffness of collagen in the vaginal wall from pregnant and nonpregnant rats, observing a mild decrease in fibril elastic modulus with pregnancy. This difference in modulus was not statistically significant.
Vaginal delivery often results in tearing to the vagina (and other pelvic structures), and the formation and propagation of such tears in vaginal tissue have been the focus of several studies by McGuire et al.65,73,76. While several studies have measured the apparent strength of vaginal tissue under uniaxial tension, McGuire et al.73 were the first to characterize the rupture strength of the intact vaginal canal by conducting free-extension inflation experiments to rupture on vaginal canals from virgin rats. They noted that the vagina was stiffer in the LD than the CD, and rupture generally occurred on the ventral wall of the vagina. The resulting tears were always aligned along the LD, indicating that rupture occurred due to the higher stresses in the CD. McGuire et al. also investigated the tear behavior in vaginal tissue in sows65 using planar biaxial testing on samples with pre-imposed tears aligned in both the LD and CD. The vagina was highly resistant to tear propagation up to stretches of 1.15, beyond which tears aligned in the CD increased in size significantly more than tears aligned in the LD65. McGuire et al.76 studied the tear propagation in vaginal tissue from virgin rats by indirectly inflating vaginal canals with pre-imposed axially-aligned tears using coaxial intraluminal latex tubes. Using this experimental setup, the authors observed a slow progression of tear propagation up to maximum pressure. Beyond this threshold, tear growth accelerated, and the pressure required for continued propagation decreased.
Simulated birth injuries and their treatment have been studied using virgin rats. Alperin et al.27 were the first to do so, using uniaxial tensile testing in the LD to assess the effectiveness of acellular scaffolding in aiding the recovery of the rat vagina 4 weeks after injury imposed via a balloon catheter. They observed that injured vaginal tissue was less stiff and weaker than uninjured tissue. Treatment via collagen scaffolding was only somewhat effective in restoring the stiffness and strength of the injured tissue, as the authors found no statistically significant differences in the mechanical properties between the injured treated group and the uninjured or injured untreated groups. In contrast, Paul et al.84 observed that six weeks after an injury imposed by pressurizing the vagina with a urethral catheter, injured tissues without treatment had significantly increased stiffness relative to uninjured tissues as measured using atomic force microscopy. Treatment via the delivery of mesenchymal stem cells was found to effectively reverse this, bringing the nanoscale stiffness of the injured vaginal tissue back to pre-injury levels. Recently, Janssen et al.63 measured the effect of an injury caused by a urethral dilator after 3 weeks using planar biaxial testing, observing decreases in stiffness in both the LD and CD, and a reduction in the degree of tissue anisotropy. These researchers found that certain regenerative treatments effectively restored the tissue’s mechanical properties to approximately match those before injury. Mesenchymal stem cell treatments and regenerative therapies promoted different aspects of vaginal tissue regeneration after injury, with the former restoring the tissue’s tangent modulus to pre-injury levels, and the latter increasing the tissue’s anisotropy index (a measure of the tissue’s anisotropy).
Parity
While the impact of pregnancy on the mechanical properties of the vagina appears to be consistent across differing animal models and testing modalities, the long-term effects of parity are not as clear (Fig. 6). Two studies have attempted to elucidate how parity alters the biomechanical properties of vaginal tissue in humans. Both Martins et al.38 and Gilchrist et al.29 conducted univariate analysis on the effects of parity on the mechanical behavior of vaginal tissue under uniaxial tensile testing. These studies did not compare nulliparous women with parous women but rather examined groups with varying degrees of parity. Women were grouped based on parity numbers, with parity levels 2 and 3 arbitrarily chosen to represent increased parity. In women of greater parity, Martins et al.38 observed significant increases in stiffness and strength of the anterior vaginal wall but not the posterior vaginal wall in the LD. Gilchrist et al.29 noted increases in stiffness and strength with parity that were not statistically significant, although the anatomical direction in which they conducted testing was not reported29,38.
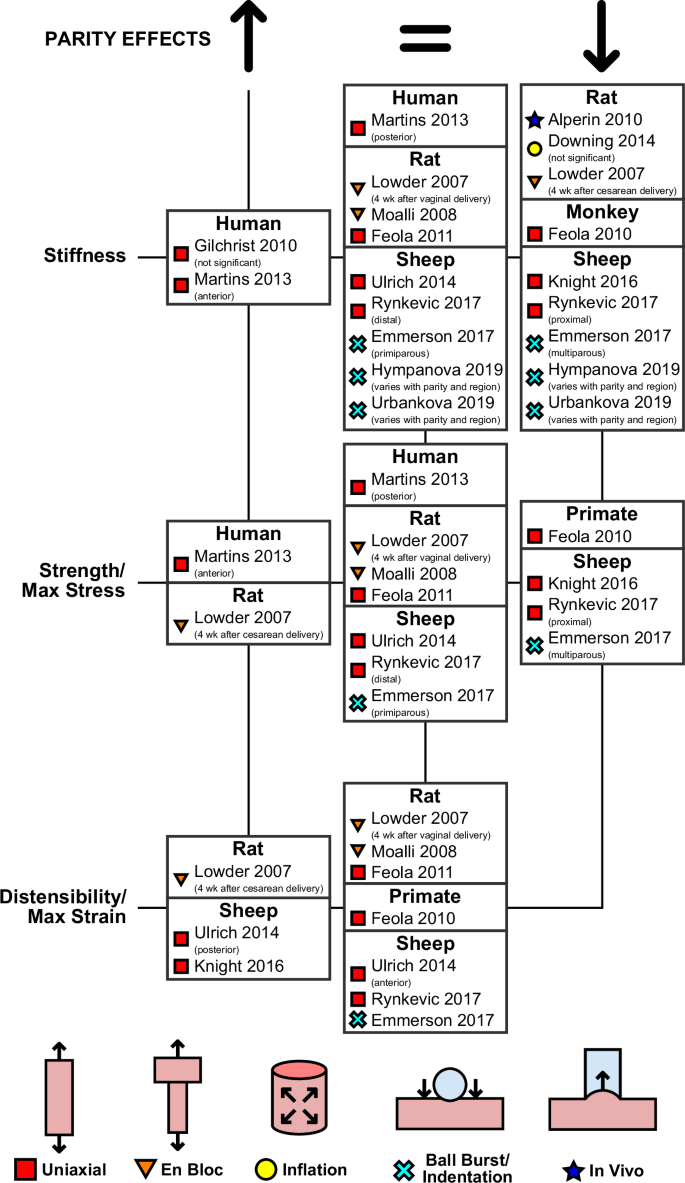
Only the first authors of the studies are reported, with the full list of authors provided in the list of references.
The majority of research on parity has been conducted in animal models, with early studies being conducted in rats using the en bloc tensile testing and uniaxial tensile testing methods. As part of their aforementioned study on the effects of pregnancy and delivery, Lowder et al.59 characterized the vagina and the supportive tissues of primiparous rats four weeks after their first delivery. They reported tissue complexes collected from rats who underwent cesarean delivery were stronger, less stiff, and more distensible than tissue complexes from virgin rats, but no differences were noted in the mechanical behavior between virgin animals and those who underwent vaginal delivery. Using the same experimental method, Moalli et al.61 mirrored the post-vaginal delivery findings, measuring no significant differences between the vaginal with the supportive tissues dissected from parous and virgin rats. As the en bloc method does not isolate the mechanical response of the vagina, other testing on rat tissue has been conducted using the uniaxial tensile testing method. In their study on the effects of pregnancy and delivery, Feola et al.34 also conducted uniaxial tensile testing in the LD of vaginal tissue collected from primiparous rats 4 weeks after vaginal delivery. The postpartum tissue exhibited similar mechanical behaviors to virgin tissue.
In contrast with the above findings, studies using in vivo and ex vivo inflation have noted long-term mechanical changes in the rat vagina with parity. As a continuation of their aforementioned study on the effects of pregnancy, Alperin et al.89 noted that the parous vagina withstood greater volume infusion during in vivo balloon catheterization than the virgin vagina, and was more compliant. Downing et al.72 observed similar behavior under ex vivo free-extension inflation. The vagina from parous rats was less stiff than the vagina from virgin rats, although their results were not statistically significant (and compared only two specimens in each group).
All the other published studies on the impact of parity have been conducted on vaginal tissue from ewes, with the majority being performed using uniaxial tensile testing in the LD. The only exception is the study on non-human primates, rhesus macaques, that was conducted by Feola et al.28. These authors noted that nulliparous vaginal tissue was significantly stronger and stiffer than parous tissue, with the parous group including results from rhesus macaques with and without prolapse.
Other studies on vaginal tissue collected from ewes have examined parity-induced changes in specific anatomical regions. When testing specimens collected from the ventral and dorsal regions of the vagina, Ulrich et al.42 noted that the parous vaginal tissue had none of the regional variations in elastic modulus, strength, or maximum strain that were present in the virgin tissue. In virgin tissue, the ventral vagina was stiffer, stronger, and less distensible than the dorsal vagina, but the parous vagina had no notable differences in mechanical properties between the ventral and dorsal regions. However, the differences in the tangent modulus, maximum strain, and strength between the different regions of the virgin vagina were not statistically significant, and no statistically significant differences existed in uniaxial tensile strength, tangent modulus, or maximum strain between parous and virgin vaginal tissue.
Knight et al.45, in their comparison of vaginal tissue collected from the dorsal vagina, noted that parous tissue had a lower tensile strength and elastic modulus and reached higher ultimate strains than nulliparous tissue. Rynkevic et al.47 also analyzed regional variations in the virgin and parous ovine vaginal walls, testing the proximal and distal regions of the vagina. The authors observed that tissue collected from the proximal region of the vagina experienced decreases in elastic modulus and maximum stress with parity, but observed no changes in the distal vagina with parity.
Testing conducted using ball-burst testing methods also indicates that parity changes the mechanical properties of vaginal tissue in ewes. However, these differences may only occur within specific ranges of deformation and stress. Emmerson et al.78 observed that the stiffness and strength of vaginal tissue from nulliparous ewes was similar to that of primiparous ewes. However, these measures decreased nearly 40% in multiparous ewes. Using the same technique, Hympanova et al.79 and Urbankova et al.80 measured the stiffness of ewe vaginal tissue. In their studies, they observed that the distal vagina of primiparous ewes had decreased stiffness compared to that of nulliparous ewes at low stresses, while there were no differences in the stiffness of vaginal tissue between multiparous and nulliparous ewes in either the distal or middle vagina at low stresses. At higher stresses, the mid-region of the primiparous vagina was significantly less stiff than that of the multiparous vagina.
Prolapse, risk factors, and treatments
The impact of prolapse on vaginal tissue mechanics has been extensively studied, primarily using human tissues collected after surgical procedures (Fig. 7). The first ex vivo experimental studies were conducted via uniaxial tensile testing on strips of vaginal tissue. Under uniaxial testing with superimposed oscillations, Lei et al.57 observed that the tangent modulus of vaginal tissue in the CD significantly increases with prolapse in both premenopausal and postmenopausal women. They also observed that prolapsed tissue has decreased strength and distensibility relative to non-prolapsed tissue. Furthermore, these results were correlated with prolapse severity, as tissue collected from patients with mild prolapse was more compliant, stronger, and more distensible than tissue collected from patients with moderate or severe prolapse.
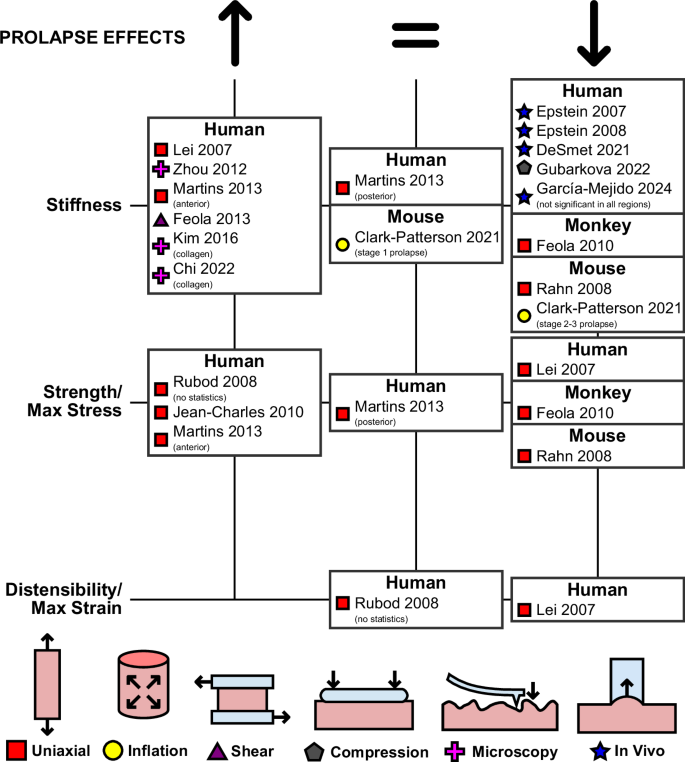
Only the first authors of the studies are reported, with the full list of authors provided in the list of references.
Other uniaxial tensile studies of the prolapsed and non-prolapsed human vagina have been conducted using quasi-static loading protocols. Rubod et al.24 characterized the strength and distensibility of tissue collected from the anterior vaginal wall in the LD, noting that prolapsed and non-prolapsed tissues reached comparable strains at rupture, but prolapsed tissue withstood more stress before rupture. Using the same protocols, Jean-Charles et al.30 characterized the changes with prolapse in both the anterior and posterior vaginal walls. Both anatomical regions experienced significant increases in stiffness in the LD at both low and high applied stresses. Martins et al.38 also compared the properties of tissue collected in the LD from both the anterior and posterior vaginal walls. They observed that prolapse was associated with increased stiffness and strength in the anterior vaginal wall, but not the posterior. Furthermore, in the anterior vaginal wall, the increases in stiffness and strength were correlated with the severity of prolapse, as tissues collected from patients with stage 3 prolapse were stiffer and stronger than tissues collected from patients with stage 1 or stage 2 prolapse.
Other ex vivo methods have also been employed to characterize the prolapsed and non-prolapsed human vagina. Feola et al.82 conducted single-lap shear testing, performing cyclic loading at various deformation rates to determine the rheologic properties of vaginal tissue. In premenopausal women, tissue with prolapse was found to be far stiffer than tissue without prolapse, with all viscoelastic measures of the stiffness under linear single-lap shear testing (complex modulus, storage modulus, and loss modulus) increasing after prolapse. Unlike other ex vivo studies, Gubarkova et al.83 found that human vaginal tissue with prolapse was more compliant than vaginal tissue without prolapse using compression optical coherence elastography.
These observations of increasing tissue stiffness with prolapse have also been replicated using mechanical testing at a microscopic level. Using scanning haptic microscopy, Zhou et al.88 observed that vaginal tissue from premenopausal women with no prolapse was less stiff than vaginal tissue from both premenopausal and postmenopausal women with prolapse. Studies using atomic force microscopy have correlated these increases in stiffness with prolapse to the stiffness of individual collagen fibrils. Kim et al.86 reported the stiffness of collagen fibrils increased with prolapse. Chi et al.87 reported the same while correlating the increasing stiffness of collagen fibrils with the increasing severity of prolapse.
In contrast to ex vivo findings, in vivo testing of the human vagina has found that vaginal tissue softens with prolapse. Epstein et al.90 compared the pressure required to achieve a 1.5 mm uplift of a section of the vaginal wall in women, with prolapse and without prolapse, using a cutometer-like device. The tissue in women with no prolapse required greater pressure to uplift, thus indicating that it was stiffer than prolapsed tissue. Epstein et al. then took this further91, observing a correlation between the severity of prolapse in women (as measured via POP-Q exam102) and the compliance of the tissue. De Smet et al.103 similarly observed that vaginal tissue in women with prolapse was more compliant than vaginal tissue in women with no prolapse when conducting in vivo testing with a vaginal manipulator device. Most recently, using shear wave elastography, García-Mejido et al.95 noted significant decreases in the stiffness of the vagina in several regions with prolapse. They used multiple regression to control for the external factors of age, menopause, and parity, which varied significantly between the prolapsed and non-prolapsed groups.
Only two animal models, non-human primates and mice, have been used to investigate the effects of prolapse. In rhesus macaques, prolapse occurs spontaneously similar to humans. Using this animal model, Feola et al.28 found the vaginal tissue of parous animals without prolapse was stiffer and stronger than the tissue of parous animals with prolapse under uniaxial tensile testing in the LD. In mice, prolapse occurs as a result of genetic mutations such as lysyl oxidases-like 1 deficiency (LOXL1−/−)104, null mutations in the gene encoding fibulin-5 (Fbln5−/−), or fibulin-5 haploinsufficiency (Fbln5+/−)105. Rahn et al.52, using an incremental loading uniaxial tensile testing protocol, observed that vaginal tissue from wild-type mice reached higher maximum stresses and was stiffer than the tissues from Fbln5−/− mice without prolapse. Tissues from Fbln5−/− mice with prolapse were even less stiff and reached even lower stresses than tissues from Fbln5−/− mice without prolapse and wild-type mice without prolapse. Clark-Patterson et al.69 also noted that fibulin-5 mutation and prolapse affected the stiffness of the murine vaginal canal. Under extension-inflation, vaginal canals from Fbln5−/− mice with severe prolapse were less stiff than vaginal canals from wild-type mice and Fbln5−/− mice with mild prolapse.
While LOXL1−/− and Fbln5−/− mutations lead to the development of prolapse in mice, these mutations can also impact the mechanics of vaginal tissue before prolapse occurs. In an en bloc uniaxial study by Alperin et al.60, the LOXL1−/− mutation was shown to significantly decrease the strength of the pelvic floor complex, and to a lesser extent increase the tissue’s extensibility, compared to young and old wild-type mice. Clark-Patterson et al.74 also measured the influence of genetic mutation independent of prolapse when they investigated the influence of fibulin-5 haploinsufficiency (Fbln5+/−), which does not lead to prolapse development in mice as quickly as full Fbln5−/− null mutations, on the creep properties of murine vaginal tissue under inflation. While they did not observe any statistically significant differences in the creep behavior, the vaginal canals of wild-type mice experienced greater increases in creep strain with pressure than the vaginal canals of the haploinsufficient mice.
To understand the etiology of prolapse, mechanical investigations of vaginal tissue have also considered common risk factors of this disorder. Aside from pregnancy and parity, two of the most significant risk factors associated with prolapse in humans are increased age and weight106,107. The effects of these factors on the mechanical properties of human vaginal tissue have been analyzed using uniaxial tensile testing. Chantereau et al.39 demonstrated that the stiffness of vaginal tissue from women with no prolapsed in the LD, at both low and high applied stresses, increased with age. Using the same testing method, Lopez et al.43 observed that vaginal tissue excised from patients with prolapse and a body mass index above 25 (i.e., overweight) was stiffer and stronger than tissue excised from patients with prolapse and a lower body mass index. These results were partially replicated by Martins et al.38, who conducted univariate analysis investigating the impacts of weight and age. They observed that the anterior vaginal wall was significantly stiffer and stronger in older patients than in younger patients. They did not measure such changes in the posterior vaginal wall, and in both regions found that weight had no significant effect on vaginal tissue biomechanics.
The influence of age and weight on vaginal tissue mechanics has also been studied using animal models. Using inflation, White et al.70 observed that murine vaginal tissue became stiffer with both increasing age and body mass. White et al.70,71 also correlated the increasing stiffness with weight and age with a decrease in the elastin content of the tissue. Elastase treatment, which degrades elastin within the tissue, was found to significantly increase the stiffness of vaginal tissue collected from young mice, with the extent of this effect decreasing in older mice. The influence of age on vaginal tissue mechanics was also studied in swine by Hakim et al.48 using uniaxial testing and optical coherence elastography testing. In contrast with other studies of age, they observed a decrease in stiffness with increasing age. While uniaxial testing revealed the vaginal tissue of piglets was significantly stiffer than the vaginal tissue of adult swine in both the LD and CD, Hakim et al. observed no significant differences in tissue mechanics with age under optical coherence elastography testing.
Given the high incidence of prolapse, many treatment methods have been evaluated by quantifying the mechanical properties of vaginal tissue. For example, Gilchrist et al.29 and Khaja et al.40, conducted uniaxial tensile testing on vaginal tissue from patients scheduled for prolapse corrective surgeries. The former study collected tissues from patients set to undergo anterior colporrhaphy repair, a procedure which tightens the anterior muscular supports near the bladder to correct prolapse, while the latter collected tissues from patients scheduled for anterior vaginal wall and vaginal vault suspension. Neither group observed a significant correlation between any mechanical testing measurements performed before corrective surgery and the long-term outcomes of the procedures.
Several treatments, however, do have notable effects on the stiffness of vaginal tissue. Using their cutometer-like device, Epstein et al.92 noted that the vaginal tissue of humans in vivo was stiffer following the prolapse corrective sacrocolpopexy procedure, a surgical intervention which utilizes mesh to lift the prolapsed organs into place, thus regaining some of the stiffness the tissue naturally has in vivo before prolapse90,91. Using optical coherence elastography, Gubarkova et al.83 also measured a partial recovery of stiffness in prolapsed vaginal tissue after pre-operative treatment with neodymium laser. This is a non-ablative laser exposure technique that induces wound healing in tissue by generating controlled thermal damage108. However, not all prolapse treatments have restorative effects. Feola et al.77 utilized ball burst testing to examine the impact of mesh implantation on the biomechanics of vaginal tissue in non-human primates. Meshes are used as a standard treatment method for prolapse to reinforce the vagina and surrounding pelvic floor tissues mechanically. The authors found that the tissue’s mechanical contribution significantly deteriorated following the implantation of Gynemesh PS polypropylene mesh but did not change significantly following the implantation of other meshes.
Menopause
Menopause has numerous effects on women, including changes to the biomechanics of the vagina16 (Fig. 8). Ex vivo studies of human tissue, conducted with uniaxial tensile testing protocols with superimposed sinusoidal vibrations, have found that menopausal vaginal tissue is stiffer than that of premenopausal tissue. Both Ettema et al.55 and Goh56 observed significant increases in the elastic moduli in menopausal tissue at multiple levels of applied stress in the LD. Lei et al.57 extended these results to tissue in the CD, finding menopause stiffens vaginal tissue in women with and without prolapse.
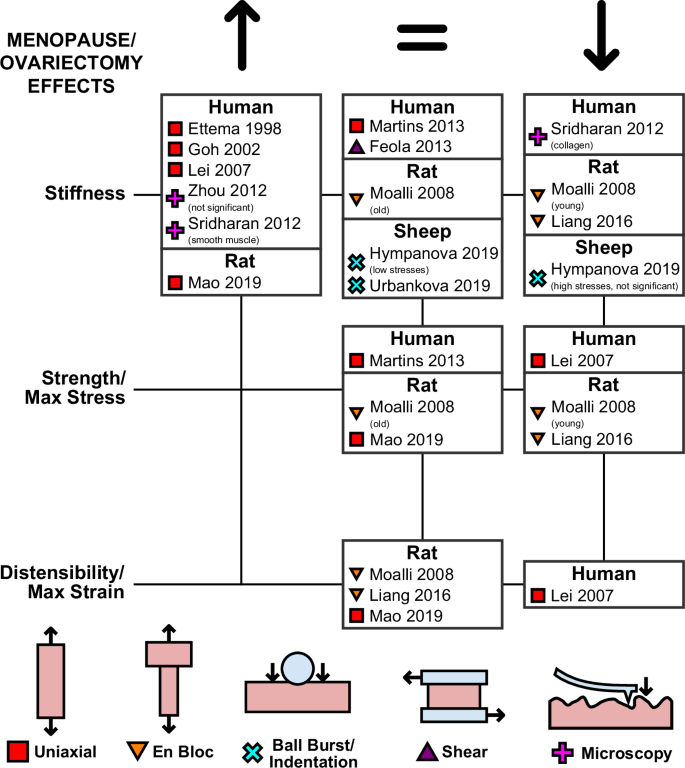
Only the first authors of the studies are reported, with the full list of authors provided in the list of references.
Other ex vivo studies on the effects of menopause on human vaginal tissue have had conflicting results. In their aforementioned uniaxial tensile testing study, Martins et al.38 conducted univariate analysis to analyze the effects of menopause, observing no significant changes in the strength or stiffness of vaginal tissue. Feola et al.82 also noted no significant changes in the rheological stiffness between premenopausal and postmenopausal vaginal tissue from prolapsed women under single-lap shear testing. Utilizing scanning haptic microscopy, Zhou et al.88 observed that vaginal tissue from menopausal women with prolapse is stiffer than vaginal tissue collected from premenopausal women with prolapse. However, their observations were not statistically significant. Using atomic force microscopy, Sridharan et al.85 compared the stiffness of collagen fibers and smooth muscle content before and after menopause. They measured that the collagen fibrils within the muscularis and mucosa of human vaginal tissue decreased after menopause. In contrast, the smooth muscle content of the muscularis became stiffer with menopause.
Most mammals do not naturally have an extended menopausal period and instead have lifelong estrous cycles109. To study the impact of menopause on vaginal tissue in animal models, its effects are frequently recreated via ovariectomy, the surgical removal of the ovaries. Investigations into the effects of ovariectomy on the vagina in rodents have been conducted using uniaxial tensile testing and en bloc tensile testing protocols. Mao et al.49 observed that ovariectomy in mice resulted in increasing stiffness of the vaginal tissue in the LD, with no significant changes in distensibility or strength, compared to non-ovariectomized controls. In contrast with Mao et al., both Moalli et al.61 and Liang et al.62, using the en bloc testing method, observed decreases in the strength and stiffness of the vagina with the supportive tissue in rats after ovariectomy. While Liang et al. conducted their comparisons in only young rats, Moalli et al. compared the effects of ovariectomy in both young and middle-aged rats, observing a significant decline in strength and stiffness only in the young group.
Ovariectomized sheep have also been used to study the impacts of menopause via two ball-burst testing studies by Hympanova et al.79 and Urbankova et al.80. In both studies, comparisons between ovariectomized multiparous sheep and multiparous sheep without ovariectomy revealed no changes in the stiffness of the tissue at low applied stresses in tissues collected from both the mid and distal regions of the vaginal canal. While Hympanova et al. did measure a decrease in the high-stress stiffness in vaginal tissue from ovariectomized to non-ovariectomized tissue animals. However, these differences were not statistically significant.
The decreased levels of estrogen following menopause can eventually lead to vulvovaginal atrophy, the incidence of which can lead to multiple detrimental effects on the vagina including vaginal dryness, irritation, postcoital bleeding, and pain110,111. Pákozdy et al.96 used compression elastography to characterize vulvovaginal atrophy, and observed that the nonatrophied vagina was more elastic than the atrophied vagina, with atrophy diagnosed using the vaginal maturation value112. While the differences they measured were statistically significant, Pákozdy et al. were not able to control for other demographics in their study, and the average age and menopausal status of patients differed significantly between the atrophic and non-atrophic groups.
The use of estrogen supplementation and hormone therapy is common to alleviate the symptoms of menopause113, and one study has been conducted in human tissue analyzing the biomechanical effect of such treatments on the vagina. As part of their single-lap shear study of the human vagina’s rheological properties, Feola et al.82 compared postmenopausal prolapsed tissue collected from women who were on hormone therapy and comparable tissue collected from women who had not received such treatment. They observed that the vaginal tissue from women who had received hormone therapy was significantly stiffer in two of the three rheological metrics (complex modulus and storage modulus) than tissue from women without hormone therapy, and the third rheological metric (loss modulus) also saw not statistically-significant increases from the untreated to the treated group.
More studies on the effects of estrogen supplementation and hormone therapy treatments on vaginal tissue after ovariectomy have been completed in rodent models. Both Moalli et al.61 and Liang et al.62 furthered their study of the effects of ovariectomy in rats by studying the effects of hormonal treatment on the pelvic block during en bloc testing. Moalli et al. found that in both virgin and parous young rats, supplementation with either estrogen, estrogen plus progesterone, or matrix metalloproteinase inhibitor resulted in the restoration of the strength and stiffness of the pelvic block, with matrix metalloproteinase inhibitor treatment restoring the mechanical properties closest to those of non-ovariectomized rats. In their study of selective estrogen receptor modulators, Liang et al. observed that while the estrogen receptor modulators were partially effective in increasing the strength and stiffness of the pelvic block to pre-ovariectomy levels, no modulator was as effective as estrogen supplementation via 17β-estradiol.
The impacts of vaginal injury on the mechanical properties of ovariectomized vaginal tissue, and the tissue’s recovery afterward have been measured using uniaxial tensile testing. In rabbits, Abramov et al.23 observed that vaginal tissue stiffness and strength were reduced after injury, but increased during recovery over the following weeks. Abramov et al.33 performed these experiments on both non-ovariectomized and ovariectomized rabbits, observing that ovariectomy in rabbits compromised the recovery of the mechanical properties of the tissue following vaginal injury. Under uniaxial tensile testing in the CD, Montoya et al.54 and Balgobin et al.53 also observed that in ovariectomized rats and guinea pigs, injured vaginal tissue was less stiff and strong than uninjured tissue. Balgobin et al. also measured the effectiveness of estrogen treatment in aiding the recovery of menopausal vaginal tissue of guinea pigs after surgical injury, and in offsetting the effects of lysyl oxidase inhibitor (an enzyme responsible for cross-linking collagen in scar formation). While injury reduced the stiffness of non-estrogenized tissue, and lysyl oxidase inhibitor reduced both the stiffness and strength of non-estrogenized tissue, estrogenized tissue saw no mechanical changes resulting from injury or treatment with lysyl oxidase inhibitor.
Discussion
The biomechanics of the vagina has garnered significant attention since the turn of the century, leading to a surge of interest in characterizing the mechanical properties of vaginal tissue. These efforts have undoubtedly advanced our knowledge of this organ and are crucial to improving clinical outcomes related to conditions such as maternal trauma, sexual dysfunction, and pelvic organ prolapse. Amidst the wealth of new research, there are several studies whose contributions to the literature, in our opinion, lack rigor. Many studies have not leveraged recent developments in experimental soft tissue mechanics, including techniques that better simulate physiologically relevant loading conditions (e.g., planar biaxial or inflation testing). Novel measurement techniques, such as digital image correlation for non-contact strain measurement65,73,75,76,114, have been underutilized. Our aim in writing this review has been to be as comprehensive and thorough as possible, and as such, we have cited and acknowledged every study that has characterized the mechanical properties of vaginal tissue. In doing so, however, we have included a number of studies whose quality and findings are, in our opinion, questionable. These studies may exhibit limitations in methodology, insufficient sample sizes, or inadequate analysis, which can undermine their findings and overall impact on the field. Rigor in future studies can be achieved through collaborative efforts between experts in the fields of mechanics and gynecology working together to achieve and continually redefine the best practices for the study of vaginal tissue biomechanics.
Uniaxial tensile testing has been the most popular method for characterizing vaginal tissue biomechanics (Fig. 2). The vast majority of uniaxial tensile testing studies have investigated the properties of vaginal tissue in the LD alone, with comparatively few characterizing these properties in the CD. While there is great value in testing the tissue in the LD, as the vagina supports the other pelvic floor organs axially, the study of the mechanical properties of the CD must not be neglected. After all, the tissue in the CD experiences the most stretch during the crucial physiological functions of intercourse and childbirth. Additionally, there is a critical gap in knowledge on the tissue’s material properties in the radial direction, and only one study has characterized the shear properties in the radial plane82. New experimental techniques should be applied to identify through-thickness and shear material properties since such properties will provide complete knowledge of the mechanical behavior of this organ. Opportunities abound for research initiatives in tissue mechanics to more thoroughly characterize the material properties of the vagina beyond tensile properties in the LD.
The tangent modulus is the most commonly reported measurement of vaginal tissue stiffness due to the material’s nonlinear stress-strain response. Notable differences in how tangent moduli are calculated between various research groups have compounded with the already significant natural variability in the mechanical behavior of the vagina, resulting in extreme variations in the reported tangent moduli (Fig. 4). Several studies reported taking tangent modulus measurements within the “linear region” of the stress-strain curve38,41,42,52. While the merits of different methods of calculating the stiffness of the vagina can be debated, there is no question that authors must be careful to provide full detail on how they quantify stiffness and take their respective measurements at given strains or over limited strain intervals. The majority of ex vivo tests on vaginal tissue have evaluated the tangent modulus up to high stresses (near or at its maximum strength before rupture), and more effort should be put into studies that focus on describing the mechanical behavior of vaginal tissue at lower stresses, which are physiologically more relevant.
Only a limited number of studies have characterized the vaginal tissue’s viscoelasticity, such as hysteresis/softening41,42,51, stress relaxation31,50,64, and creep74,75,81,115 phenomena. Although the time-dependent mechanical properties of vaginal tissue are critical to many physiological functions, several authors have emphasized their importance, particularly during childbirth. The second stage of labor can have an extended duration116 and, for this reason, the viscoelastic properties likely govern the mechanical response of the vagina. Comparisons of vaginal tissue from pregnant and virgin rats have demonstrated that vaginal tissue from pregnant animals exhibits more significant amounts of stress relaxation31 and creep81. However, more investigations of vaginal tissue viscoelasticity are required to extend our limited knowledge, as well as investigate the changes in these properties with other life events such as parity, prolapse, and menopause. Finally, the underlying mechanisms dictating the viscoelastic behavior of the vagina have not yet been identified, and elucidating their role in the biomechanics of the vagina could help the development of innovative approaches to vaginal health treatments.
In vivo, the vagina is consistently hydrated by a continuous supply of fluids and nutrients from the body. Because of this, it is commonly accepted that tissue hydration must be maintained during ex vivo testing, to avoid the tissue drying out and affecting its mechanical properties. Several studies have shown that if and how vaginal tissue is stored and hydrated between the time of dissection and ex vivo testing can influence the mechanical properties22,26,29. Specifically, the exposure to atmospheric conditions22 and the method of tissue hydration leading up to testing26,29 alter the stiffness and strength of vaginal tissue. Beyond this, the impact of maintaining tissue hydration during testing has not been fully investigated. A great number of studies of the vagina did not report taking additional steps for maintaining tissue hydration, such as immersion in saline or Krebs bath, during ex vivo testing21,24,30,38,41,42,47,78,117. It is quite possible this impacted the measured mechanical properties of tissue, particularly in studies of relaxation or creep50,81 or cyclic loading51, where the tissue was exposed to atmospheric conditions for an extended duration. While this review, for the sake of brevity, has not discussed how tissue hydration was maintained during different studies on a case-by-case basis, future experimental work should clarify the importance of keeping vaginal tissues hydrated before and during testing, as well as the best practices for doing so.
Across various testing methods and animal models, the impact of pregnancy on the biomechanics of the vagina has been remarkably consistent. Studies on pregnancy have found that vaginal tissue from pregnant animals is more compliant, more distensible, and less strong than nonpregnant tissue (Fig. 5). Only Jing found that the vagina from pregnant rats is stronger than the vagina from virgin rats, and this difference may be accounted for by different measures of stress. Jing reported the true stress, calculating stress as the axial force per current cross-sectional area, where most studies utilize engineering stresses, in which the force is normalized by the cross-sectional area of the tissue in the reference (initial) configurations31. Regardless, most studies have only investigated the mechanics of the vagina in late pregnancy: only Lowder et al.59 and Feola et al.34 have performed mechanical testing of the tissue at multiple time points in pregnancy. Both groups observed the mechanical behavior of the rat vagina with and without supportive structures varied from mid- to late-pregnancy, but their specific results differed, likely due to their different experimental methods34,59. To clarify these findings, greater focus should be placed on characterizing the differences in the biomechanics of vaginal tissue at various stages of pregnancy moving forward. These data will be critical to the development of new non-invasive techniques that track and predict the remodeling of the vagina during pregnancy, ensuring maternal health during pregnancy, delivery, and postpartum.
In general, parity seems to reduce the stiffness and strength and increase the distensibility of vaginal tissue in animals, although there are variations and inconsistencies within these results that warrant further probing (Fig. 6). In rats, studies that utilized inflation72,89 found the parous vagina to be less stiff than the virgin vagina. At the same time, no such differences have been noted in studies using en bloc uniaxial59,61 and conventional uniaxial34 testing methods. A plausible explanation for this may be the importance of the mechanical behavior in the CD, which was not characterized by the aforementioned uniaxial studies conducted in the LD. In ewe vaginal tissue, parity seems to affect the mechanical properties differently across the various anatomical regions45,47,78,79,80. Only Ulrich et al.41 observed no significant changes in mechanical properties due to parity across all regions of the vagina. However, their results found that the parous vaginal tissue had none of the regional differences in biomechanical metrics that were evident in virgin and pregnant tissue, indicating that parity likely did impact the vagina in some way not captured in their statistical comparisons. Moreover, their study included only a small number of virgin samples (n = 3), potentially limiting their conclusions. A confounding factor in current mechanical investigations is the inadequate control of age, as in every study on the effect of parity in animals, the parous animals were older than the respective control animals. To further elucidate the impact of parity on the vaginas of different animal models, future work should use testing protocols that can distinguish the response of the vagina in multiple anatomical directions and involve a larger number of animals while controlling for confounding factors such as age. Animal models are advantageous for designing controlled experiments. However, significant differences in the function of their reproductive system can impact the extent to which results from testing vaginal tissues from animals can be translated to humans. For example, unanswered questions remain on the effect of their much larger litter size on the biomechanics of the vagina.
Notably, no study using animal models observed that vaginal tissue stiffened or strengthened with parity. These findings in animals contrast with the studies of human tissue by Gilchrist et al.29 and Martins et al.38, who both reported an increase in human vaginal tissue stiffness with parity (Fig. 6). However, there were limits to these observations: the changes noted by Gilchrist et al. were not statistically significant, while Martins et al. only observed changes in stiffness and strength in the anterior vagina and not the posterior. Furthermore, both studies were limited to univariate analysis comparing women of lesser parity to women of greater parity. In these analyses, the effects of parity cannot be fully separated from the impact of other factors such as age, weight, menopause, and prolapse. Many of these factors have been shown to impact the stiffness of human vaginal tissue under ex vivo uniaxial tensile testing24,30,38,39,43,82. As such, more experimental data are needed to determine which, if any, of the animal models may be most suitable for studying parity-induced changes in the mechanical behavior of the vagina in humans.
In humans, the results of ex vivo testing on the effects of prolapse on the elastic modulus of vaginal tissue are remarkably consistent (Fig. 7). Nearly every ex vivo study has reported an increase in the stiffness of the tissue30,38,57,82,88 and tissue’s components86,87 with prolapse. Only Gubarkova et al.83 found that the stiffness of vaginal tissue decreased with prolapse with ex vivo testing. This discrepancy may have resulted simply from a difference in methodology, as they are the only group that utilized optical coherence elastography as their experimental technique in characterizing prolapsed tissue. The general results of ex vivo testing do notably contrast the findings of in vivo testing: the results of cutometer-like devices90,91, vaginal manipulator probe103, and elastography95 have indicated that prolapse softens vaginal tissue. In addition to the different testing methods, this difference may be due to the fact that during in vivo testing, the vagina is attached to the other organs and tissues of the pelvic floor118. In contrast, during ex vivo testing, vaginal tissue is tested in isolation. Thus, it is possible that in vivo testing measures an overall softer vaginal tissue with prolapse because the vagina is in a state of decreased support. While Epstein et al. reported measurements taken at the vaginal sidewall, away from areas they estimated were likely to be directly impacted by prolapse90,91, it is very likely that the entire vagina was affected by prolapse. When interpreting in vivo studies, care should be taken before attempting to draw conclusions on the mechanical behavior of vaginal tissue specifically without considering the effect of the surrounding organs and tissues.
An in vivo method of assessing vaginal mechanics that has not been discussed in this review is vaginal tactile imaging, a form of elastography characterized by the use of a probe to apply a compressive load to a region of the vagina119. Vaginal tactile imaging has been regularly used since its inception to estimate regional vaginal elasticity through intravaginal probing. It has been used to characterize the response of the vagina to prolapse and various prolapse treatments120. The use of vaginal tactile imaging has yielded similar insights on the mechanical response of the vagina as other in vivo methods: prolapse has shown to soften the vagina in vivo121, while some reconstructive surgeries have been shown to restore the stiffness of the vagina after prolapse122,123. In addition to its use in characterizing vaginal mechanics, vaginal tactile imaging has also been used to describe the mechanics of the pelvic muscles and other nearby supportive structures124,125,126,127 and also as a means of simply assessing the health status of the vagina128,129,130. As a review on the use of vaginal tactile imaging has been recently completed by Sarvazyan et al.120, the use of this technique has not been thoroughly reviewed here.
Whereas ex vivo testing has consistently demonstrated vaginal tissue from women with prolapse is stiffer than vaginal tissue from women with no prolapse, ex vivo testing has shown that murine vaginal tissue softens with prolapse52,69 as well as with its triggering genetic mutations60,69. Softening of the vagina with prolapse was also observed in non-human primates when comparing parous animals with and without prolapse using uniaxial tensile testing28. These discrepancies with human studies may result from prolapse having a different etiology. For instance, prolapse does not occur naturally in specific animal models and is induced in mice with genetic mutations104,105. However, this explanation is less likely for non-human primates, the animal model most biologically similar to humans, which also spontaneously experience prolapse as humans do. Further research should be conducted to improve the interpretation of prolapse studies in animal models and to extend conclusions regarding the impact of prolapse on human tissues.
The impacts of menopause on both the active and passive mechanics of vaginal tissue have been reviewed recently by Gimenez et al.16 (Fig. 8). While menopause is generally believed to stiffen vaginal tissue in humans, the primary studies supporting this claim did not control for age, a factor associated with increased tissue stiffness55,56,57,85,88. Naturally, postmenopausal women were significantly older than premenopausal women38,39,70. Furthermore, very few studies of human vaginal tissue have analyzed the impact of menopause in the absence of prolapse (Fig. 4), an event that also affects the mechanical properties of vaginal tissue. Animal models provide the opportunity to control for external factors such as age. Ovariectomizing animals, as done in rats49,61,62 and ewes79,80, offer a valuable approach for controlling confounding factors while studying the effects of menopause. However, this method does not fully replicate the human experience, as it bypasses the perimenopausal phase, a critical transitional period in women. To address this limitation, alternative interventions such as the use of ovotoxins in rodents show promise in recreating the perimenopausal phase in preclinical models131.
Findings using ovariectomy in age-controlled animals have not been consistent. Hympanova et al.80 and Urbankova et al.80 observed no notable changes in the stiffness of vaginal tissue with ovariectomy when comparing multiparous sheep. Mao et al.49 observed that ovariectomy increased the stiffness of rat vaginal tissue under uniaxial tensile testing, while Moalli et al.61 and Liang et al.62 observed ovariectomy decreased the stiffness of rat tissue under en bloc uniaxial testing. Unfortunately, full comparisons between these conflicting results are difficult to draw due to the different experimental approaches of ball burst testing, uniaxial testing, and en bloc tensile testing. Furthermore, no study has measured the biomechanical effects of menopause and ovariectomy at several time points, leaving a critical knowledge gap regarding the time-dependent effects of estrogen deprivation resulting from menopause. More studies on animal models, using techniques that isolate the mechanical response of the vagina at various time points after ovariectomy or other interventions while controlling for age, will be necessary to fully elucidate the effects of menopause on the vagina, which at this point are unclear.
Numerous studies have examined the ability of the vagina to recover from life events and injuries (e.g. menopause, prolapse, or birth injury) by measuring changes in the tissue’s mechanical properties27,53,54,61,62,63,77,82,83,84,92. These properties can be used as objective indicators of tissue function, offering valuable insights into how well the vagina heals and recovers following surgical or hormonal intervention. However, not all such studies have included a relevant control group of uninjured or unaffected vaginal tissues to provide a baseline for healthy tissue function. For example, when assessing the effectiveness of estrogen for treating menopause, a control group of premenopausal patients should be included. This allows us to evaluate whether the estrogen treatment truly restores the tissue’s mechanical properties to the healthy premenopausal state. Without such comparison, the efficacy of the applied treatments in restoring the native biomechanical properties of the vagina cannot be fully assessed. While some studies on the effects of medical treatment on vaginal tissue have been presented, this review has primarily focused on presenting the impact of natural or life-altering experiences on the mechanical function of the vagina rather than on the specific outcomes of treatments. Nonetheless, mechanics-based metrics are undeniably valuable, not only for understanding physiological and pathological conditions but also for evaluating potential therapeutic approaches.
The microstructure of the human vagina has been described using a variety of histological techniques. The relative quantity and organization of key extracellular matrix components, such as elastin and collagen, have been shown to be the primary determinants of the organ’s mechanical properties. The elastin fibers of the subepithelium are extremely compliant and capable of withstanding substantial stretching without breaking132. An increased volume fraction of elastin has been linked with increased compliance of vaginal tissue in humans44,46. Collagen fibers are comparatively much stiffer than elastin, and an increased amount of collagen has been associated with increased vaginal tissue stiffness in humans83,88. Finally, smooth muscle content has been shown to primarily contribute to the active mechanical response of the vagina in rats where tissue viability can be preserved during testing15,114,133,134. In ewes, Rynkevic et al.47,117 associated an increase in the compliance of pregnant vaginal tissue relative to virgin vaginal tissue with not just relative decreases in collagen content and increases in elastin content, but also an increase in the amount of smooth muscle. However, the direct applicability of many animal models to humans has not been thoroughly examined, and caution must be exercised in extrapolating the results of animal research on the link between mechanical and structural properties to humans135.
Common lab animals such as rodents, rabbits, pigs, sheep, and non-human primates have been used in mechanical studies of vaginal tissue since their vaginas share some morphological similarities with human vaginas135. Mechanical properties of the vagina from other non-lab animals, such as dolphins, have also been measured136. However, their organs significantly differ from humans (e.g., muscular protrusions of the vaginal wall into the lumen)137. In most common lab animals, the composition and orientation of the various microstructural components of the vagina have been correlated with the mechanical behavior of this organ. For example, Ulrich et al.41 measured regional variances in collagen in postmenopausal ewes, noting that the higher concentration in total collagen content in the introitus is associated with greater tissue stiffness and strength. This aligns with human findings showing that vaginal tissue stiffness is correlated with collagen content44,83,88. Similarly, recent work by Akintunde et al.67 and White et al.70,71 has suggested that increased tissue stiffness in aging mice is linked to reduced elastin content, mirroring results in human vaginal tissue44,46. Since the correlations between the mechanics and morphology of vaginal tissue are similar in both humans and the most commonly used animal models, studies on animal tissue will not only continue to advance the development of new experimental methods but also drive the formulation of new research hypotheses, leading to breakthroughs in vaginal tissue mechanics.
With mechanical data, a few investigators have proposed the use of theoretical and computational models that can capture the results of their experimental observations on vaginal tissue25,30,32,39,44,46,50,51,67,138,139,140,141,142,143. Once fully validated, these physics-based models will be instrumental in exploring scenarios and conditions that cannot be studied experimentally due to ethical considerations (e.g., testing of vaginal tissue in pregnant women), confounding factors (e.g., spurious association between parity and age), and technical limitations (e.g., inability to test and preserve tissue for long-time intervals). Given the structural complexity of the vaginal tissues and the incomplete set of mechanical data on vaginal tissue, physics-based models represent time- and cost-efficient strategies to fill the existing gaps in our knowledge. As research efforts in women’s health continue to grow, more data on the physiology and pathology of the vagina will become available, making recent advances in data-driven and machine-learning models remarkably promising in improving women’s healthcare.
Conclusions
Increasing awareness of the importance of biomechanics in many physiological and pathological conditions has driven recent efforts to properly characterize the mechanical properties of the vagina. While some trends have been identified through various studies (Figs. 4–8), limitations in experimental methods (e.g., sample size, hydration conditions, confounding factors) and variations in methodology (e.g., tissue preparation, testing protocol, and measurement techniques) often limit our ability to make comparisons of the outcomes from different studies. When used judiciously, animal models provide great experimental control at a reduced cost, enabling more decisive conclusions from larger sample sizes with reduced variability. More research is needed to validate the use of animal models for studying various human conditions, as it is clear that not all life events affect human and animal vaginal tissues in – the same way, particularly in the cases of parity and prolapse. As progress continues in the field of biomechanics, with the advancement of experimental, theoretical, and computational methods, novel mechanical metrics that characterize vaginal tissues can be identified. By strengthening collaborations among experts in biomechanics and women’s health, these mechanical attributes can guide the development and implementation of diagnostic and treatment strategies, improving women’s physical, mental, and emotional well-being throughout their lives.


Responses