The role of ferroptosis and oxidative stress in cognitive deficits among chronic schizophrenia patients: a multicenter investigation

Introduction
Schizophrenia (SCZ) is a persistent and debilitating psychiatric condition affecting approximately 1% of the global population, posing significant health and economic burdens1. Several key challenges hinder our ability to effectively address SCZ. First and foremost, there is a lack of a definitive cure, particularly for individuals with chronic SCZ characterized by cognitive impairments as core symptoms2. These impairments, encompassing executive function, abstract thinking, attention, and memory, lead to poor medication adherence, ineffective treatment outcomes, social dysfunction, and prolonged illness. Second, managing chronic SCZ with cognitive deficits emphasizes the importance of developing early prediction and prevention strategies to prevent the progression to chronicity. However, the complex and multifaceted etiology of SCZ, involving a combination of genetic and environmental influences with intricate interplay, presents significant obstacles for accurate prediction1,3. Although neuropsychological tests can reliably assess cognitive impairments in SCZ, objective biological markers are still essential for mechanistic studies and prognostic evaluation of SCZ.
Recently, growing evidence suggested a central role for oxidative stress (OS) imbalance in the neuronal deterioration and pathogenesis of SCZ4. Redox balance is maintained by the precise control of reactive oxygen species (ROS) through redox mechanisms, with glutathione (GSH) being the primary redox system in the body. Initial observations in the 1990s detected alterations in GSH metabolism within the cerebrospinal fluid of individuals diagnosed with SCZ5. Quantification of malondialdehyde (MDA), a common marker of lipid peroxidation end-products, is frequently employed in clinical research to assess OS6. Furthermore, superoxide dismutase (SOD), an antioxidant enzyme, has been linked to the overall severity and cognitive impairments observed in individuals experiencing acute-phase SCZ2,7. These findings, along with additional discoveries from environmental and genetic research on abnormalities in mitochondrial and energy metabolism8, as well as neuroinflammation9, indicate that OS, which ultimately disrupts redox regulation, plays a pivotal role in the pathophysiology of SCZ.
Ferroptosis, an iron-dependent cell death pathway, is a significant OS mechanism, with ROS playing a central role in its reactions10. Characterized by iron overload, ROS accumulation, and lipid peroxidation, ferroptosis is triggered by excessive iron levels11. Transferrin (TF) and ferritin (FE) are crucial for regulating iron metabolism by facilitating transport, binding, and storage11. However, iron overload can induce ROS production via the Fenton reaction, leading to OS and lipid peroxidation11. Additionally, acyl-CoA synthase long-chain family member 4 (ACSL4) promotes the synthesis of phospholipids rich in polyunsaturated fatty acids, which are susceptible to lipoxygenase-mediated conversion into lipid hydroperoxides, ultimately contributing to ferroptosis-induced cell death11. Conversely, glutathione peroxidase 4 (GPX4), in conjunction with the co-factor GSH, catalyzes the conversion of lipid peroxidation peroxide bonds (L-OOH) to hydroxyl groups (L-OH), thereby inactivating peroxides and attenuating ferroptosis reactions within cells11. Sirtuin 1 (SIRT1) and nuclear factor erythroid 2-related factor 2 (Nrf2) act as upstream regulators in ferroptosis-mediated OS responses, and their dysfunction has been linked to depression12. Furthermore, research suggests a connection between ferroptosis and cognitive decline in neurodegenerative disorders13. In SCZ, studies have shown decreased mRNA expression of the antioxidant xc– system subunits solute carrier family 3 member 2 (SLC3A2) and solute carrier family 7 member 11 (SLC7A11) in peripheral blood leukocytes14. These proteins are now recognized as key regulators of ferroptosis. Additionally, in vitro experiments suggest that Human endogenous retroviruses (HERVs) can induce ferroptosis by suppressing the expression of GPX4 and SLC3A215. Building on this prior research, we hypothesized that ferroptosis may contribute to the development of SCZ. Our study aimed to investigate the potential link between ferroptosis and the manifestation of psychiatric symptoms and cognitive impairments in chronic SCZ patients.
Chronic SCZ patients with prolonged illness exhibit a more stable disease state and poorer prognosis compared to those experiencing a first episode and drug-naive SCZ. As a result, they hold greater importance for therapeutic development, and their disease course is more representative of the overall SCZ population. This study investigated ferroptosis-related parameters in chronic SCZ patients with cognitive deficits. We explored two key questions: (1) Are there alterations in plasma biomarkers related to ferroptosis in SCZ patients with cognitive deficits? (2) Is there a correlation between ferroptosis-related biomarkers and cognitive function/psychiatric symptoms in these patients? Ultimately, we aimed to identify biological markers that can enhance our understanding of disease mechanisms and potentially serve as prognostic indicators in SCZ patients with cognitive deficits.
Materials and methods
Study design and participants
This research employed an observational, cross-sectional, retrospective design conducted between October 2023 and April 2024. A total of 204 chronic SCZ patients were recruited from three hospitals: the First Affiliated Hospital of Xinjiang Medical University, the Fourth People’s Hospital of Aksu Prefecture, and the Kashgar District Kangning Hospital. All participants met diagnostic criteria for SCZ according to two independent psychiatrists using the International Statistical Classification of Diseases and Related Health Problems (ICD-10)16 and the Mini-International Neuropsychiatric Interview (MINI)17. They were excluded based on Sponheim’s criteria for recent-onset SCZ as outlined by Sponheim18. Inclusion criteria comprised: age 18–60, illness duration exceeding 5 years, stable oral antipsychotic medication for at least 12 months, no anti-inflammatory or antibiotic use within 4 weeks, completion of primary education, absence of significant visual/auditory impairments, and comprehension of investigator questions. Exclusion criteria encompassed Montreal Cognitive Assessment (MoCA) scores of 26 or higher19,20, substance use disorders (except tobacco), neurological diseases, severe/acute somatic diseases, endocrine infectious/immune system diseases, pregnancy/breastfeeding, and difficulty participating in the study.
Two hundred sixteen healthy controls (HC) were recruited from the First Affiliated Hospital of Xinjiang Medical University Physical Examination Center and nearby communities. Potential participants with biological kinship with the patients or significant visual/auditory impairments were excluded. The HC group was carefully matched to the patient group by age, gender, ethnicity, body mass index (BMI), education level, marital status, and smoking habits. All HC participants scored 26 or higher on the MoCA, indicating normal cognitive function. Two research psychiatrists conducted unstructured clinical interviews to assess the participants’ current mental state, personal medical history, and family history of psychiatric disorders. None of the participants exhibited signs of psychiatric disorders or had a family history of such conditions. Additionally, individuals meeting the aforementioned exclusion criteria were excluded from the study.
Clinical examination and cognitive assessments
A standardized data collection protocol ensured comprehensive demographic and clinical information. Through subject interviews and medical record review, researchers gathered details on age, BMI, gender, ethnicity, education, marital status, family location, and smoking history for both SCZ and HC. Additionally, the SCZ group underwent a more detailed clinical data collection, including the age of onset, illness duration, and antipsychotic medication dosage converted to chlorpromazine equivalent doses using the minimum effective dose method outlined by Woods for both first- and second-generation medications21. The average daily dose of benzodiazepines was calculated using lorazepam dose equivalents.
In this study, the psychopathology of subjects was evaluated using the positive and negative syndrome scale (PANSS)22,23. Two highly experienced psychiatrists trained in PANSS scoring consistency administered the scale. The total score inter-rater reliability exceeded 0.8 throughout the study. PANSS assesses psychopathology through three subscales: positive symptoms (delusions, disorganization, suspiciousness), negative symptoms (emotional withdrawal, poor rapport, abstract thinking difficulties), and general psychopathology (anxiety, depression, poor attention, disturbance of volition). Higher scores reflect greater illness severity. Additionally, anxiety and depression were assessed using the self-reported 7-item generalized anxiety disorder scale (GAD-7) and a 9-item patient health questionnaire (PHQ-9), both well-validated tools with higher scores indicating increased symptom severity24,25,26.
The MoCA is a widely used screening tool in clinical and research settings. With a maximum score of 30 points, a score below 26 indicates cognitive impairment19,20. The MoCA’s strength lies in its comprehensive assessment of various cognitive domains through eight sections: visuospatial abilities, executive functions, attention, memory, concentration, language, verbal abstraction, and orientation. Higher scores reflect better cognitive functioning. Compared to other assessment tools, the MoCA offers a more in-depth evaluation. Prior research has established the MoCA’s validity as a concise screening instrument for detecting cognitive impairment in individuals with schizophrenia aged 18 and over27,28,29. In this study, two specially trained psychiatrists administered and evaluated all MoCA assessments.
Laboratory measurements
In a fasted state, venous blood samples were collected from all participants between 7:00 a.m. and 9:00 a.m. using ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes. Following centrifugation at 3000 rpm for 15 min, plasma was isolated and stored at −80 °C for subsequent analysis. All measurements were performed in duplicate by two blinded technicians at an independent laboratory. Plasma levels of GPX4 (EK14343, Signalway Antibody, Greenbelt, MD, USA), ACSL4 (ELK8993, ELK Biotechnology Co., Ltd., Wuhan, China), TF, FE, SIRT1, and Nrf2 (E-EL-H6028, E-EL-H0168, E-EL-H1546, E-EL-H1564; Elabscience Biotechnology Co., Ltd., Wuhan, China) were quantified using the enzyme-linked immunosorbent assay (ELISA) kit as per manufacturer instructions. Colorimetric assays were employed to determine GSH and iron levels, while thiobarbituric acid and hydroxylamine assays were used to assess MDA and SOD levels, respectively (A006-1-1, A003-1, A001-1, A039-1-1; Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Detailed information regarding units, detection ranges, and coefficients of variation for each indicator is provided in Supplementary Table 1.
Statistical analysis
A power analysis using G*Power software (version 3.1.9.7) determined a minimum sample size of 89, based on a significance level of 0.05, a power of 0.95, and a medium effect size (0.15) for multivariate linear regression. The study exceeded this requirement with 204 chronic SCZ and 216 HC participants. All statistical analyses were performed using SPSS 29.0 (IBM Corporation), and visualizations were created with GraphPad Prism 10. The normality of data distribution was confirmed using Q–Q plots and the Shapiro–Wilk test. Descriptive statistics were employed to report baseline characteristics, with continuous variables presented as mean ± standard deviation (SD) and categorical variables presented as frequencies and percentages (%). Independent samples t-tests were used for comparisons between groups for continuous variables, while chi-square or Fisher’s exact test was used for categorical data. Pearson’s correlation assessed associations between numerical measures. Furthermore, multiple linear regression analyses were conducted to investigate potential relationships between ferroptosis and OS markers with PANSS (psychiatric symptom severity) and MoCA (cognitive function). Plasma ferroptosis-related biomarkers (ACSL4, GPX4, GSH, TF, FE, Iron, SIRT1) and OS markers (SOD, MDA) were included as independent variables, with PANSS and MoCA as dependent variables. All P-values were two-tailed, with a significance level of 0.05.
Ethics approval and consent to participate
All participants or their legal guardians provided written informed consent after a thorough explanation of the study protocol and potential risks. Data were anonymized to ensure participant confidentiality. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (240424-14) and adhered to the tenets of the Declaration of Helsinki.
Results
Demographic and general clinical data
Table 1 summarizes the demographic characteristics and general clinical data of the SCZ and HC groups. There were no significant differences between the groups in terms of age, gender, ethnicity, BMI, education level, marital status, family location, or smoking habits (all P > 0.05). For the SCZ participants, the mean age of onset was 22.43 ± 3.20 years, with a mean disease duration of 21.94 ± 7.13 years. Participants administered a mean chlorpromazine equivalent dose of 592.84 ± 164.90 mg/day and a lorazepam equivalent dose of 1.58 ± 0.70 mg/day.
Clinical characteristics of participants
In the SCZ group, patients exhibited a mean PANSS total score of 79.18 ± 11.07, with positive symptoms at 16.63 ± 3.43, negative symptoms at 26.71 ± 4.31, and general psychopathology symptoms at 35.84 ± 8.16. The PHQ-9 scores, GAD-7 scores, MoCA total scores, and scores of all sub-indexes for the 204 SCZ and 216 HC participants are presented in Table 2 and Supplementary Table 2, along with their respective means and standard deviations. Compared to the HC group, the SCZ group showed significantly lower MoCA total scores and all subscale scores (P < 0.001), indicating poorer cognitive function. Additionally, they exhibited higher levels of anxiety and depression (P < 0.001).
Plasma ferroptosis and OS parameters
Figure 1a–j and Supplementary Table 3 summarize ferroptosis and OS-related indices in blood samples from both SCZ and HC participants. Significantly lower levels of GPX4 [(6.78 ± 2.24) ng/mL vs (13.28 ± 3.49) ng/mL, P < 0.001, Fig. 1a], GSH [(5.58 ± 1.57) g/L vs (6.12 ± 1.31) g/L, P < 0.001, Fig. 1b], Iron [(52.27 ± 23.03) μmol/L vs (61.31 ± 23.72) μmol/L, P < 0.001, Fig. 1c], TF [(2.23 ± 0.74) mg/mL vs (2.45 ± 0.67) mg/mL, P = 0.002, Fig. 1d], SIRT1 [(0.85 ± 0.41) ng/mL vs (1.08 ± 0.46) ng/mL, P < 0.001, Fig. 1e], and Nrf2 [(2.50 ± 0.76) ng/mL vs (2.94 ± 0.84) ng/mL, P < 0.001, Fig. 1f] were observed in the SCZ group compared to the HC group. Conversely, levels of ACSL4 [(7.33 ± 2.98) ng/mL vs (5.47 ± 1.59) ng/mL, Fig. 1g] and FE [(0.49 ± 0.23) ng/mL vs (0.33 ± 0.17) ng/mL, Fig. 1h] were significantly higher in the SCZ group (all P < 0.001). Regarding OS markers, plasma SOD [(22.26 ± 5.56) U/mL vs (16.36 ± 5.37) U/mL, Fig. 1i] and MDA [(12.27 ± 1.66) nmol/mL vs (8.87 ± 1.79) nmol/mL, Fig. 1j] were significantly elevated in the SCZ group compared to the HC group (all P < 0.001).
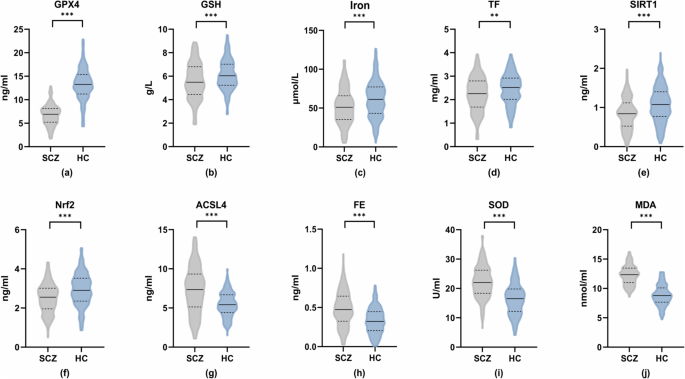
Panels (a) through (j) depict the concentration levels of the following biomarkers in both groups: a glutathione peroxidase 4 (GPX4), b glutathione (GSH), c iron, d transferrin (TF), e sirtuin 1 (SIRT1), f nuclear factor erythroid 2-related factor 2 (Nrf2), g acyl-CoA synthetase long-chain family member 4 (ACSL4), h ferritin (FE), i superoxide dismutase (SOD), and j malondialdehyde (MDA). **P < 0.01; ***P < 0.001.
Association between ferroptosis and OS markers
Within the SCZ group, the correlation analysis presented in Table 3 revealed several significant relationships between ferroptosis and OS markers. ACSL4 exhibited negative correlations with SIRT1 (r = −0.23, P < 0.001) and Nrf2 (r = −0.17, P < 0.05), while showing positive correlations with SOD (r = 0.18, P < 0.05) and MDA (r = 0.23, P < 0.001). Conversely, GPX4 demonstrated positive correlations with SIRT1 (r = 0.19, P < 0.01) and Nrf2 (r = 0.17, P < 0.05), and negative correlations with SOD (r = −0.14, P < 0.05) and MDA (r = −0.33, P < 0.001). Additionally, GSH displayed a positive correlation with SIRT1 (r = 0.19, P < 0.01) and a negative correlation with MDA (r = −0.29, P < 0.001), but no significant correlations with SOD and Nrf2 (P > 0.05). TF showed no significant associations with SIRT1, Nrf2, or SOD (P > 0.05) but displayed a negative correlation with MDA (r = −0.21, P < 0.01). Similarly, FE lacked significant correlations with SIRT1, Nrf2, and MDA (P > 0.05) but showed a positive correlation with SOD (r = 0.18, P < 0.01). Finally, iron levels were not significantly associated with any of the other markers (SIRT1, Nrf2, SOD, and MDA; P > 0.05).
Correlation of psychiatric symptoms and negative emotions with indicators related to ferroptosis and OS
Within the SCZ group, Pearson’s correlation analysis was conducted to examine relationships between ferroptosis and OS markers with PANSS and its subscale scores, depression (PHQ-9), and anxiety (GAD-7) scores (Figs. 2–4 and Supplementary Table 4). The total PANSS score showed positive correlations with plasma ACSL4 (r = 0.19, P < 0.01), FE (r = 0.19, P < 0.01), SOD (r = 0.24, P < 0.001), and MDA (r = 0.21, P < 0.01) levels [Fig. 2a–d], and negative correlations with GPX4 (r = −0.23, P < 0.001), GSH (r = −0.28, P < 0.001), TF (r = −0.20, P < 0.01), and iron levels (r = −0.16, P < 0.05) [Fig. 2e–h]. Similar patterns of correlations were observed for negative symptoms, with positive correlations for ACSL4 (r = 0.31, P < 0.001), FE (r = 0.24, P < 0.001), and MDA (r = 0.21, P < 0.01) [Fig. 3a–c], and negative correlations for GPX4 (r = −0.31, P < 0.001), GSH (r = −0.25, P < 0.001), TF (r = −0.24, P < 0.001), and SIRT1 (r = −0.16, P < 0.05) [Fig. 3d–g]. General psychopathology symptoms also exhibited positive correlations with ACSL4 (r = 0.15, P < 0.05) and FE (r = 0.14, P < 0.05) levels, and a positive correlation with SOD (r = 0.23, P < 0.001) [Fig. 4a–c]. Conversely, negative correlations were observed for GSH (r = −0.23, P < 0.001), TF (r = −0.17, P < 0.05), and iron (r = −0.19, P < 0.01) [Fig. 4d–f]. iron level was negatively correlated with positive symptoms (r = −0.17, P < 0.05), while other indices did not show significant correlations with positive symptoms (P > 0.05). Depression (PHQ-9) scores only showed a negative correlation with GSH (r = −0.16, P < 0.05), while neither PHQ-9 nor GAD-7 scores exhibited significant correlations with any other ferroptosis or OS markers (P > 0.05). No significant differences in plasma ferroptosis and OS markers from anxiety and depression in the HC Group (Supplementary Table 5, P > 0.05).
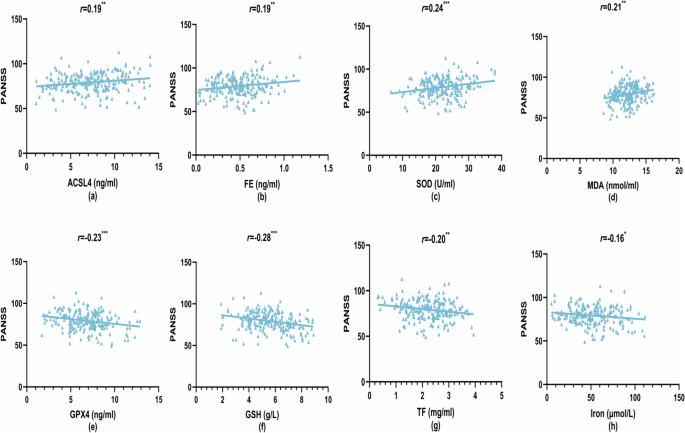
a Acyl-CoA synthetase long-chain family member 4 (ACSL4) levels vs PANSS scores, b ferritin (FE) levels vs PANSS scores, c superoxide dismutase (SOD) levels vs PANSS scores, d malondialdehyde (MDA) levels vs PANSS scores, e glutathione peroxidase 4 (GPX4) levels vs PANSS scores, f glutathione (GSH) levels vs PANSS scores, g transferrin (TF) levels vs PANSS scores, and h iron levels vs PANSS scores. *P < 0.05; **P < 0.01; ***P < 0.001.
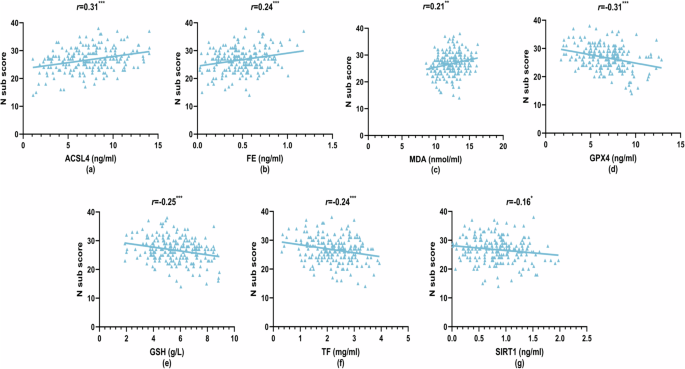
a Acyl-CoA synthetase long-chain family member 4 (ACSL4) levels vs N sub scores, b ferritin (FE) levels vs N sub scores, c malondialdehyde (MDA) levels vs N sub scores, d glutathione peroxidase 4 (GPX4) levels vs N sub scores, e glutathione (GSH) levels vs N sub scores, f transferrin (TF) levels vs N sub scores, and g sirtuin 1 (SIRT1) levels vs N sub scores. *P < 0.05; **P < 0.01; ***P < 0.001.
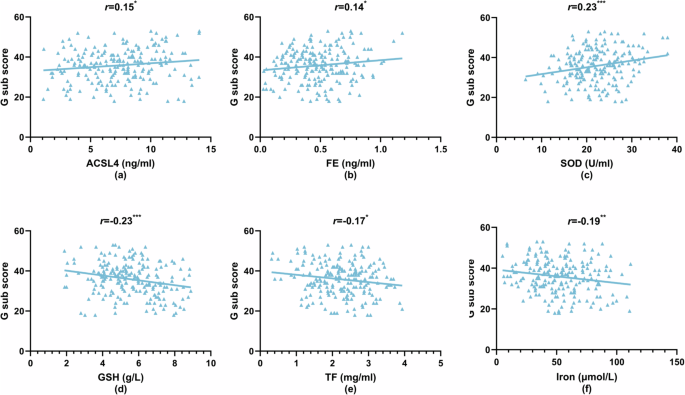
a Acyl-CoA synthetase long-chain family member 4 (ACSL4) levels vs G sub scores, b ferritin (FE) levels vs G sub scores, c superoxide dismutase (SOD) levels vs G sub scores, d glutathione (GSH) levels vs G sub scores, e transferrin (TF) levels vs G sub scores, and f iron levels vs G sub scores. *P < 0.05; **P < 0.01; ***P < 0.001.
Correlation of cognitive performance with indicators related to ferroptosis and OS
First, in the HC group, the results showed no significant correlation between ferroptosis and OS indicators and cognitive function (Supplementary Table 5, P > 0.05). Then, In the SCZ group, there were no significant differences in cognitive function based on gender, age, antipsychotic medications, or benzodiazepine use (Supplementary Tables 6 and 7, P > 0.05). Finally, analysis of the data in Supplementary Table 8 and Fig. 5a–h revealed significant correlations between ferroptosis and OS markers with MoCA total score and its subscale scores. MoCA total score showed positive correlations with GPX4 (r = 0.33, P < 0.001), GSH (r = 0.29, P < 0.001), TF (r = 0.25, P < 0.001), and SIRT1 (r = 0.19, P < 0.01) levels, while negative correlations were observed with ACSL4 (r = −0.34, P < 0.001), FE (r = -0.25, P < 0.001), SOD (r = −0.24, P < 0.001), and MDA (r = −0.24, P < 0.001) [Fig. 5a–h]. Similar patterns of correlations emerged for MoCA subdomains. Visuospatial and executive function scores positively correlated with GSH (r = 0.28, P < 0.001) and iron levels (r = 0.21, P < 0.01). Attention scores showed positive correlations with GSH (r = 0.18, P < 0.01) and SIRT1 (r = 0.17, P < 0.05). Language function scores positively correlated with GPX4 (r = 0.24, P < 0.001) and TF (r = 0.15, P < 0.05). Abstraction scores showed a positive correlation with GPX4 (r = 0.31, P < 0.001) and negative correlations with ACSL4 (r = −0.21, P < 0.01), SOD (r = −0.25, P < 0.001), and MDA (r = −0.17, P < 0.05). Delayed memory scores positively correlated with GPX4 (r = 0.21, P < 0.01) and negatively correlated with ACSL4 (r = −0.20, P < 0.01) and MDA (r = −0.22, P < 0.01). Orientation only negatively correlated with ACSL4 levels (r = −0.14, P < 0.05).
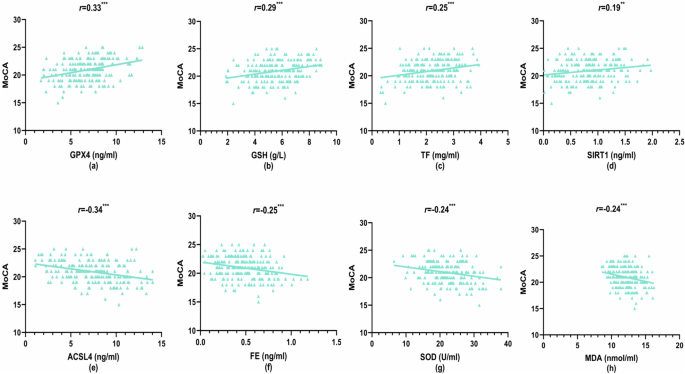
a Glutathione peroxidase 4 (GPX4) levels vs MoCA scores, b glutathione (GSH) levels vs MoCA scores, c transferrin (TF) levels vs MoCA scores, d sirtuin 1 (SIRT1) levels vs MoCA scores, e acyl-CoA synthetase long-chain family member 4 (ACSL4) levels vs MoCA scores, f ferritin (FE) levels vs MoCA scores, g superoxide dismutase (SOD) levels vs MoCA scores, and h malondialdehyde (MDA) levels vs MoCA scores. **P < 0.01; ***P < 0.001.
Multiple linear regression analysis of psychiatric symptoms
Three multiple linear regression models were developed to investigate the relationships between ferroptosis and OS markers with PANSS total score, negative symptoms, and general psychopathology symptoms (dependent variables). The models included statistically significant parameters from the correlation analysis as independent variables (Tables 4 and 5 and Supplementary Table 9). For the total PANSS score, the regression analysis revealed a negative association with GSH (β = −0.15, P = 0.030) and iron (β = −0.13, P = 0.043), a positive association with SOD (β = 0.17, P = 0.015), and no significant associations with ACSL4, GPX4, FE, TF, and MDA (P > 0.05; Table 4). Negative symptoms exhibited positive regression coefficients with ACSL4 (β = 0.18, P = 0.007) and FE (β = 0.19, P = 0.004), and a negative coefficient with GPX4 (β = −0.19, P = 0.006). No significant associations were observed with GSH, TF, SIRT1, or MDA (P > 0.05; Table 5). Conversely, general psychopathology symptoms showed negative associations with GSH (β = −0.15, P = 0.031) and iron (β = −0.17, P = 0.014), a positive association with SOD (β = 0.18, P = 0.009), and no significant associations with ACSL4, FE, or TF (P > 0.05; Supplementary Table 9).
Multiple linear regression analysis of cognitive performance
A multiple linear regression model was employed to investigate the association between ferroptosis and OS markers with cognitive function in the SCZ group, using the MoCA total score as the dependent variable. The model included age and statistically significant parameters from the correlation analysis as independent variables (ACSL4, GPX4, GSH, FE, TF, SIRT1, SOD, and MDA). The results revealed that lower GPX4 levels (β = 0.19, P = 0.005) and higher levels of ACSL4 (β = −0.18, P = 0.007) and FE (β = −0.16, P = 0.012) were significantly associated with lower MoCA scores, indicating greater cognitive dysfunction. No significant associations were observed with the remaining markers (P > 0.05; Table 6). Similar regression analyses were conducted for each MoCA subscale. Abstraction displayed a positive correlation with GPX4 (β = 0.24, P < 0.001) and a negative correlation with SOD (β = −0.20, P = 0.003) (Supplementary Table 10). Language function only showed a significant positive correlation with GPX4 (β = 0.21, P = 0.003) (Supplementary Table 11). No significant correlations were found between delayed memory and MDA, ACSL4, or GPX4 (all P > 0.05) (Supplementary Table 12). Visuospatial and executive function scores showed positive correlations with GSH and iron levels (β = 0.26, P < 0.001; β = 0.18, P = 0.008) (Supplementary Table 13). Finally, attention scores displayed positive correlations with both GSH (β = 0.16, P = 0.028) and SIRT1 (β = 0.15, P = 0.033) (Supplementary Table 14).
Discussion
Circulating molecular indicators may serve as valuable tools for understanding disease mechanisms and monitoring disease progression in SCZ patients with cognitive deficits. These biological markers are particularly promising due to their convenient sampling, standardized detection, and potential for extensive clinical application in mechanistic and longitudinal studies. The objective of this research was to explore the correlation between specific ferroptosis and OS-related biomarkers and clinical manifestations of SCZ patients with cognitive deficits. Building upon prior investigations into OS and clinical manifestations in SCZ30,31, this study, grounded in the framework of ferroptosis, has established for the first time a link between ferroptosis and both psychiatric symptoms and cognitive performance in individuals with chronic SCZ. To ascertain the potential correlation between these variables, it is imperative to mitigate confounding factors and biases. We incorporated eight general demographic variables, including age, gender, and BMI, among others, and observed no significant differences between the two groups. Furthermore, cognitive function and levels of anxiety and depression were evaluated utilizing the MoCA, PHQ-9, and GAD-7 scales in both the SCZ and HC cohorts. Following the establishment of equivalent baseline data between the SCZ patients and the HC group, we identified notable variances in cognitive function levels and emotional states of anxiety and depression.
Measurement of indices related to ferroptosis and OS in SCZ and HC groups
The study compared ferroptosis and OS markers in the blood plasma of individuals with chronic SCZ and HC. Patients with SCZ exhibited significantly lower levels of the ferroptosis markers GPX4, GSH, TF, iron, SIRT1, and Nrf2 than the HC group. Conversely, individuals with SCZ showed significantly higher levels of ferroptosis and OS markers ACSL4, FE, SOD, and MDA.
Our study identified elevated plasma levels of the OS markers MDA and SOD in patients with SCZ, aligning with findings from Uddin et al.32, Guidara et al.33, and Hursitoglu et al.34. This heightened OS can damage cell membranes and degrade polyunsaturated fatty acids, leading to MDA accumulation, a significant factor in SCZ pathophysiology. Elevated SOD levels might be a compensatory response to excess ROS generated during OS. However, conflicting reports exist. Yang et al.6 found reduced levels of both MDA and SOD, while Buosi et al.35 documented elevated MDA and diminished SOD. Despite these discrepancies, numerous studies point towards altered OS parameters in SCZ, suggesting its potential role in the disorder’s pathophysiology. Differences in analytical techniques, biological samples, medication use, and disease severity across studies might contribute to these inconsistencies, making direct comparisons challenging.
Our study found significantly lower SIRT1 and Nrf2 levels in the SCZ group compared to the HC group, suggesting potential dysregulation of OS at an earlier stage. This aligns with previous reports linking the SIRT1 gene to schizophrenia36 and reduced plasma SIRT1 levels in SCZ patients37. Moreover, animal studies have shown that38 clozapine, an antipsychotic medication, can normalize abnormal behaviors in mice by enhancing the Nrf2-dependent glutathione synthesis system induced by phencyclidine. SIRT1, an NAD+-dependent deacetylase, modulates various cellular pathways by influencing substrates like NF-κB and Nrf237. Nrf2 is crucial for maintaining cellular redox balance, regulating inflammatory responses, and protecting cells from oxidative damage38. These findings suggest that the SIRT1/Nrf2 pathway might be involved in the OS mechanisms underlying SCZ, potentially by bolstering the body’s antioxidant defenses against oxidative damage caused by specific toxic agents.
Our investigation into ferroptosis markers aimed to elucidate its distinct contribution to SCZ beyond OS. The SCZ group displayed elevated levels of FE and ACSL4, alongside diminished levels of TF, GPX4, iron, and GSH compared to the HC group. Interestingly, while plasma iron levels were decreased, we observed elevated levels of FE and TF, suggesting a complex dysregulation of iron homeostasis. Our finding of decreased plasma iron levels is consistent with a recent meta-analysis of 39 studies involving 5151 subjects, which demonstrated significantly lower iron levels in SCZ patients across different clinical stages and treatment conditions39. The relationship between systemic and tissue iron levels in SCZ appears to be complex. Previous postmortem studies have revealed increased iron levels in the prefrontal cortex of SCZ patients, accompanied by decreased FE levels, suggesting a disruption in iron-FE coupling40. This apparent discrepancy between peripheral and central iron levels suggests a complex dysregulation of ferroptosis rather than a simple deficiency state. Furthermore, studies indicate that elevated FE levels are typically linked to increased intracellular iron storage, which may provide substrates for ferroptosis. Excessive iron reserves can induce the release of ROS and promote OS. Additionally, these reserves may participate in ferroptosis through autophagy41, potentially contributing to the cognitive impairments observed in patients with SCZ. Our findings of decreased GSH levels in the SCZ group concur with a recent meta-analysis42. GSH scavenges free radicals and removes lipid peroxidation byproducts. Intriguingly, bioinformatic analyses suggest potential differences in GPX4 and ACSL4 expression between SCZ and healthy individuals15,43. However, these findings require clinical validation. GPX4, a GSH-dependent enzyme, repairs oxidative damage to lipids, while ACSL4 promotes ferroptosis by generating lipid hydroperoxides11. The observed disparities in GPX4 and ACSL4 levels between the groups suggest that ferroptosis may play a significant role in SCZ etiology.
In SCZ, ferroptosis is strongly associated with OS
Ferroptosis is a form of regulated cell death mediated by iron-dependent lipid peroxidation and OS, characterized by excessive accumulation of free cytosolic iron, leading to lipid peroxidation and OS11. Our study findings suggest a significant interplay between ferroptosis and OS in SCZ. SOD levels displayed a positive association with ACSL4 and FE levels, but a negative correlation with GPX4 levels. MDA levels positively correlated with ACSL4 but negatively correlated with GPX4, reduced GSH, and TF levels. SIRT1 levels positively correlated with GPX4 and GSH, while negatively correlated with ACSL4. Nrf2 exhibited a positive correlation with GPX4 and a negative correlation with ACSL4. Emerging evidence from neurodegenerative diseases44,45 and related disorders, such as depression46, highlights a growing connection between ferroptosis and OS. Ferroptosis is characterized by iron overload, lipid peroxide accumulation, and ROS production11. However, limited clinical research has explored ferroptosis in SCZ. Our findings propose that in SCZ, upstream regulators of OS, SIRT1, and Nrf2, might influence the ferroptosis pathway. GPX4 inactivation leading to GSH depletion, alongside high ACSL4 activation and iron overload, likely contributes to OS and lipid peroxidation. This subsequent OS intensifies cellular ferroptosis, potentially increasing susceptibility to SCZ. Our findings of altered ferroptosis-related proteins suggest potential involvement of the ferroptosis pathway in SCZ. However, to establish ferroptosis as a definitive pathogenic mechanism, further evidence of ferroptosis-specific cell death is needed, beyond the observed changes in ferroptosis-related proteins. Future research should focus on validating ferroptotic cell death in post-mortem brain tissue, investigating the causal relationship between these markers and cell death using cell models, and evaluating the therapeutic potential of targeting these pathways. These studies would help establish whether GPX4, ACSL4, and other ferroptosis-related proteins could serve as viable therapeutic targets for SCZ.
Ferroptosis and OS-related markers are associated with psychiatric symptoms and cognitive deficits in SCZ
Schizophrenia exhibits a phenotypic heterogeneity, primarily characterized by positive symptoms (hallucinations, delusions), negative symptoms (social withdrawal, blunted affect), and cognitive impairments. Current treatment approaches diverge based on symptom profiles, with antipsychotics primarily targeting positive symptoms but offering limited efficacy for negative and cognitive dysfunction. This necessitates further investigation into clinical symptoms with a potential link to ferroptosis. Additionally, identifying ferroptosis markers associated with cognitive decline in SCZ would be valuable for future research.
Initially, independent samples t-tests were conducted to examine gender differences in cognitive functioning among patients with SCZ. The analysis revealed no statistically significant differences between male and female patients in either the total cognitive function scores or any of the cognitive subscale measures (all P > 0.05). Then, we performed correlation analyses to assess the relationships between cognitive function and age as well as medication usage (antipsychotics and benzodiazepines). These analyses demonstrated no significant associations between cognitive performance and either age or medication use (all P > 0.05), suggesting that these factors did not significantly influence cognitive outcomes in our sample. Subsequently, our study employed multiple linear regression analysis to demonstrate that, within the model, elevated levels of ACSL4 and FE, alongside decreased levels of GPX4, were indicative of worsened negative symptoms and cognitive function in patients, as opposed to OS markers. Furthermore, higher GPX4 levels were protective in specific cognitive domains like abstraction and language. Prior research has established the significant impact of the ferroptosis signaling pathway on cognitive function in Alzheimer’s disease13 and Parkinson’s47. However, the exact mechanisms by which ferroptosis influences negative symptoms and cognitive deficits in SCZ remain unclear. A study using a nationally representative sample of elderly individuals in the United States revealed a significant correlation between elevated serum FE concentrations and impaired cognitive function during task performance48. FE plays a crucial role in regulating intracellular iron levels and cell death, particularly through its involvement in redox processes. Ferritinophagy refers to the targeted autophagic degradation of FE, leading to the accumulation of cytoplasmic iron, primarily in the form of Fe2+49. Elevated iron levels can trigger oxidative damage and lipid peroxidation, ultimately leading to ferroptosis. Studies using mouse embryonic fibroblasts have shown that the absence of the ACSL4 gene prevents ferroptosis induction50, suggesting its potential as a biomarker for predicting ferroptosis onset. Another study in rats with early brain injury from subarachnoid hemorrhage demonstrated that suppressing the ACSL4 gene improved OS, behavior, and cognitive impairments51. Within the ferroptosis pathway, the xCT/GSH/GPX4 axis acts as the primary negative regulator. GPX4, the key downstream regulator and the main known ferroptosis inhibitor, plays a crucial role. Hambright et al.52 found that deleting GPX4 in mice resulted in significant hippocampal neuron degeneration and cognitive decline. Li et al.53 demonstrated that berberine, by suppressing the RSL3-induced ferroptosis pathway, could enhance cognitive function in a mouse model of Alzheimer’s disease. A recent meta-analysis exploring the link between negative symptoms and neurocognitive function revealed a moderate correlation between negative symptom scores and various cognitive domains54. Notably, disorganized communication, impoverished language and thought processes related to verbal memory, and motor impoverishment overlapping with working memory were particularly linked55. This suggests an overlap between negative symptoms and cognition in SCZ, with ferroptosis potentially acting as a contributing mechanism. These findings provide a theoretical framework for identifying novel, symptom-specific therapeutic targets for pharmacological intervention.
This study further identified a link between lower GSH and iron levels, elevated SOD levels, and the severity of both overall psychiatric symptoms and general psychopathology in individuals with SCZ. GSH and iron levels positively correlated with visuospatial and executive function scores. Previous research has established a strong association between OS markers and both clinical symptoms and cognitive function in SCZ2,6,56. Xu et al.57 observed reduced iron levels in the gray matter of first-episode SCZ patients, particularly those with more severe symptoms. Yang et al.2 a direct relationship between plasma GSH activity and visuospatial/structural cognitive function. Conversely, Chen et al.58 documented a negative association between elevated SOD levels and attentional performance in chronic SCZ. Additionally, a randomized controlled trial demonstrated that the antioxidant L-carnosine improves executive function in SCZ59, supporting our findings. These observations suggest that various signaling pathways, including the ferroptosis pathway (characterized by changes in GSH and iron), might contribute to both the emergence of psychiatric symptoms and visuospatial/executive function deficits in SCZ. While multiple pathways can induce OS, the specific role of ferroptosis in SCZ remains understudied. Further investigations are warranted to validate our findings.
Our findings suggest that ferroptosis-related biomarkers, particularly GPX4, ACSL4, and FE, hold promise for detecting cognitive impairments and negative symptoms in SCZ. Additionally, PANSS total scores and general psychopathology symptoms displayed significant correlations with levels of GSH, iron, and SOD. These findings raise the possibility of utilizing these biomarkers for diagnosis and treatment monitoring in SCZ. However, the multifaceted nature of psychiatric symptoms and cognitive impairments in SCZ suggests the potential involvement of other contributing factors. Future research is warranted to further validate these findings and explore the broader role of ferroptosis in SCZ.
Limitation
This study has certain limitations that should be acknowledged. Firstly, as a cross-sectional design, it cannot definitively establish causality between the psychiatric symptoms, cognitive deficits, and ferroptosis in SCZ. Future research should incorporate larger-scale longitudinal clinical studies to address this. Secondly, while we statistically controlled for confounding factors, the potential influence of disease progression, medication use, and other unmeasured variables cannot be ruled out, particularly in patients with extended hospitalizations. Therefore, further investigation into the relationship between ferroptosis and SCZ in first-episode patients or those not receiving medication is warranted. Thirdly, the current study focused on patients with MoCA scores below 26, and future research would benefit from including SCZ patients with minimal cognitive deficits (MoCA 27–30) to provide a more comprehensive understanding of the relationship between ferroptosis markers and cognitive function in SCZ. Finally, the correlation between plasma ferroptosis parameters and corresponding changes within the central nervous system remains unclear. Additional research utilizing cell models, animal models, and human brain imaging techniques is crucial to elucidate this relationship.
Conclusion
Our initial investigation suggests a significant role for ferroptosis in the psychiatric symptoms and cognitive impairments of SCZ. Biomarkers like GPX4, ACSL4, and FE are associated with negative symptoms and cognitive deficits, while GSH, iron, and SOD correlate with general psychopathology and overall psychiatric manifestations. Within specific cognitive domains, GPX4 abnormalities may be linked to impairments in abstraction and language, while GSH activity deviations could be connected to visuospatial and executive function deficits. In conclusion, we propose that ferroptosis and OS might be significantly correlated with the psychiatric symptoms and cognitive impairments observed in chronic SCZ patients. However, further research is necessary to elucidate the underlying mechanisms. Despite the ongoing challenge of identifying reliable SCZ biomarkers, continued biological investigations hold promise for novel insights that could enhance our understanding of disease mechanisms and improve prognostic and therapeutic strategies in clinical settings.




Responses